Table 1.
Optimization of Catalytic Reaction Conditionsa
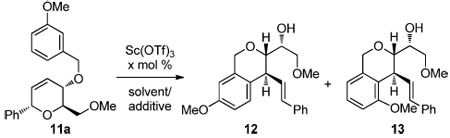 | |||
|---|---|---|---|
| entry | x | solvent/additive (equiv) | % yield (12:13)b |
| 1 | 25 | CH2Cl2 / none | 72; 5:1c |
| 2 | 20 | MeNO2 / none | 94; 3.7:1 |
| 3 | 10 | MeNO2 / Bu4NPF6 (2) | 97; 3.3:1d |
| 4 | 2 | MeNO2 / Bu4NPF6 (2) | 85; 2.8:1 |
| 5 | 20 | MeNO2 / Bu4NPF6 (0.2) | 93; 3.6:1d |
| 6 | 0 | MeNO2 / Bu4NPF6 (2) | -e |
| 7 | 0 | MeNO2 / TfOH (0.2) | 87; 2.5:1d |
| 8 | 20 | MeNO2 / Bu4NPF6 (0.2)/ DTBMP (1) |
-e |
| 9 | 20 | MeNO2 / Bu4NPF6 (0.2)/ 3 Å MS |
-e |
All reactions conducted at 0 °C to rt for 2 h unless otherwise noted.
Isolated yield and ratios.
rt, 17 h.
time = 30 min.
no reaction. DTBMP = 2,6-di-tert-butyl-4-methylpyridine.
