Table 2.
Dihydropyran Rearrangements Resulting from Friedel-Crafts Alkylationa
| entry | substrate | product (% yield)b | entry | substrate | product (% yield)b |
|---|---|---|---|---|---|
 |
 |
9 | 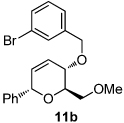 |
 |
|
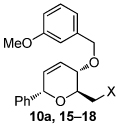
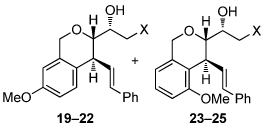
| 1 | X= OH (10a) | 83; (19:23 = 2.8:1) | 10 | 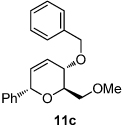 |
 |
| 2 | OTBS (15) | 95; (19:23 = 5.5:1; X = OH) | |||
| 3 | OAc (16) | 98; (20:24 = 2:1) | |||
| 4 | Br (17) | 93; (21) | |||
| 5 | N3 (18) | 80; (22:25 = 4.5:1) | |||
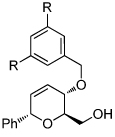 |
 |
11 | 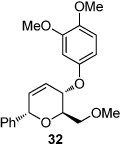 |
 |
|
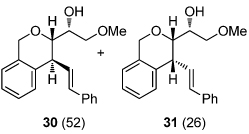
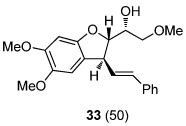
| 6 | R = OMe (10b) | 26 (97) | 12 | 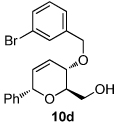 |
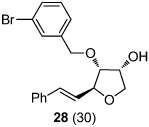 |
| 7 | R = Me (10c) | 27 (81) | |||
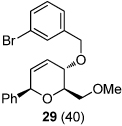
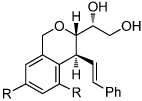
| 8 | 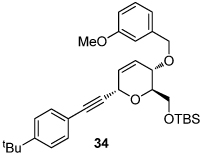 |
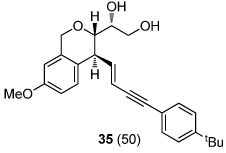 |
Conditions: Sc(OTf)3 (20 mol %), Bu4NPF6 (20 mol %), CH3NO2, 0 °C to rt, 30 min.
Isolated yield and ratio.
