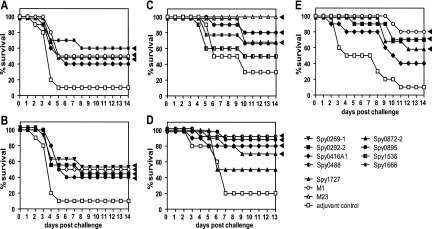
FIG. 2.
Protection by candidate antigens in intravenous and intranasal challenge models. CD-1 (A and B), BALB/c (C and D), and NMRI (E) mice (10 mice/group) were immunized subcutaneously (A, B, E) or intranasally (C, D) either with 50 μg M1 or M23 protein (positive control) or with 50 μg candidate antigens with 1% aluminum hydroxide (A, B), IC31 (10 nmol KLK, 0.4 nmol ODN1a) (C), 0.5 μg of MALP-2 (D), or CFA-IFA (E). For the negative control, PBS with adjuvant was used. About 2 weeks after the last booster immunization, mice were challenged intranasally with 1 × 108 CFU S. pyogenes A20-MA (M23) (A, B), intranasally with 7 × 105 CFU S. pyogenes A20-MA (C), intranasally with 7.6 × 107 CFU S. pyogenes A147-MA (M106) (D), or intravenously with 7.5 × 107 CFU S. pyogenes AP-1 (M1) (E). Numbers of surviving mice are plotted as a percentage of total mice. Statistically significant differences based on the Kaplan-Meier log rank (Mantel-Cox) test are indicated by arrowheads.