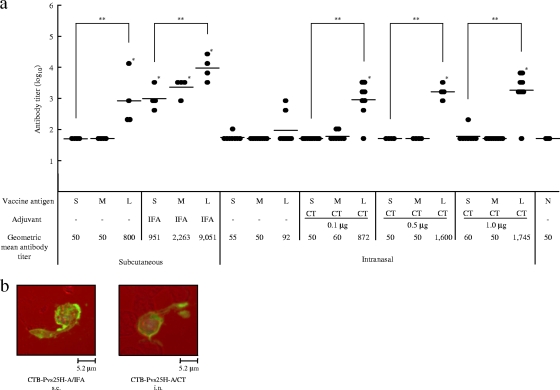
FIG. 3.
Immunogenicity of the CTB-Pvs25H-A fusion protein for induction of a Pvs25-specific serum IgG response. (a) Female BALB/c mice (four or eight mice per group) were immunized with Pvs25H-A alone (30 μg) (S), a mixture of cholera toxin B subunit (CTB; 30 μg) and Pvs25H-A (30 μg) (M), or the CTB-Pvs25H-A fusion protein (60 μg), by the subcutaneous (s.c.) or the intranasal (i.n.) route (L), three times, at weeks 0, 2, and 3. Serum samples were collected a week after the third immunization and were evaluated for Pvs25-specific IgG titers. All mice received the same amount of Pvs25H-A antigen, i.e., 30 μg per injection. IFA and CT at various doses (0.1 to 1.0 μg) were used as s.c. and i.n. vaccine adjuvants, respectively. Nonimmune serum (N) was used as a negative control. Antibody titers were defined as the serum dilution that gave an OD415 of 0.1 or the serum dilution for which a one-point-higher dilution (2-fold) gave an OD415 of <0.1. *, Significantly different from nonimmune serum as determined by the Wilcoxon-Mann-Whitney test (P < 0.05); **, significantly different among the three groups (S, M, and L) as determined by the Kruskal-Wallis test (P < 0.001). (b) Ookinete-specific reactivity of induced antisera analyzed by immunofluorescence. The antisera derived from s.c. immunization with the CTB-Pvs25H-A fusion protein emulsified with IFA (CTB-Pvs25H-A/IFA), or the fusion protein administered i.n. with CT (1 μg) (CTB-Pvs25H-A/CT) specifically recognized native Pvs25 protein expressed on the surface of Plasmodium vivax ookinetes. Scale bar, 5.2 μm.