Abstract
The protozoan parasite Leishmania donovani, the etiological agent of visceral leishmaniasis, is renowned for its capacity to sabotage macrophage functions and signaling pathways stimulated by activators such as gamma interferon (IFN-γ). Our knowledge of the strategies utilized by L. donovani to impair macrophage responsiveness to IFN-γ remains fragmentary. In the present study, we investigated the impact of an infection by the amastigote stage of L. donovani on IFN-γ responses and signaling via the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway in mouse bone marrow-derived macrophages. The levels of IFN-γ-induced expression of major histocompatibility complex class II and inducible nitric oxide synthase (iNOS) were strongly reduced in L. donovani amastigote-infected macrophages. As the expression of those genes is mediated by the transcription factors STAT1α and IFN regulatory factor 1 (IRF-1), we investigated their activation in amastigote-infected macrophages treated with IFN-γ. We found that whereas STAT1α protein levels and the levels of phosphorylation on Tyr701 and Ser727 were normal, IRF-1 expression was inhibited in infected macrophages. This inhibition of IRF-1 expression correlated with a defective nuclear translocation of STAT1α, and further analyses revealed that the IFN-γ-induced STAT1α association with the nuclear transport adaptor importin-α5 was compromised in L. donovani amastigote-infected macrophages. Taken together, our results provide evidence for a novel mechanism used by L. donovani amastigotes to interfere with IFN-γ-activated macrophage functions and provide a better understanding of the strategies deployed by this parasite to ensure its intracellular survival.
Protozoan parasites of the genus Leishmania cause a spectrum of diseases in humans, ranging from self-healing ulcers to potentially fatal visceral leishmaniasis, which affect millions of people worldwide. These parasites are transmitted to mammals under their promastigote form upon the blood meal of infected sand flies. Following their phagocytosis by macrophages, promastigotes differentiate into amastigotes, the mammalian stage of the parasite which resides and replicates inside phagolysosomes. To ensure their survival within macrophages, Leishmania parasites have evolved strategies to sabotage signaling pathways that modulate host cell microbicidal properties (8, 14, 19, 20, 26). Hence, Leishmania-infected macrophages are characterized by the abnormal expression of activation-associated functions and by an unresponsiveness to activators such as gamma interferon (IFN-γ) (2, 9, 12, 17, 25, 33, 34).
The signaling events initiated by IFN-γ have been investigated in great detail (29, 36). Binding of IFN-γ to its multisubunit receptor triggers tyrosine phosphorylation of the IFN-γ receptor-associated kinases Janus kinase 1 (JAK1) and JAK2, which in turn phosphorylate the transcription factor signal transducer and activator of transcription 1α (STAT1α) on residue Tyr701. Tyrosine-phosphorylated STAT1α molecules then homodimerize and are further phosphorylated on Ser727, an important step for acquisition of full transcriptional activity (7, 42). STAT1α homodimers interact with importin-α5, a member of the karyopherin-α family of nuclear localization signal receptors (23, 37). This interaction with importin-α5 is central to the nuclear translocation of STAT1α and the subsequent transcriptional regulation of IFN-γ responsive genes, such as IFN regulatory factor 1 (IRF-1) and class II transactivator (CIITA), which regulate the expression of genes involved in microbial killing and antigen presentation (such as inducible nitric oxide synthase [iNOS] and major histocompatibility complex class II [MHC II]) (6, 18, 40).
Studies aimed at understanding the mechanisms by which Leishmania donovani impairs IFN-γ-induced macrophage responses revealed that this parasite targets distinct steps along the JAK-STAT pathway. Hence, within minutes, L. donovani promastigotes activate the protein tyrosine phosphatase SHP-1, causing the tyrosine dephosphorylation of JAK2 (5, 12). Furthermore, L. donovani promastigotes cause an abnormal nuclear translocation of STAT1α which is related to its rapid proteasome-mediated degradation (11). In addition, L. donovani promastigotes downregulate the IFN-γ receptor α-chain protein levels (28) and induce the transient expression of the suppressor of cytokine signaling 3 (SOCS3), which negatively regulates IFN-γ signaling (3). Thus, as they initiate infection, L. donovani promastigotes efficiently shut off the predominant signaling cascade of one of the most important macrophage activators.
Similar to promastigotes, L. donovani amastigotes inhibit IFN-γ-induced responses in macrophages, including the expression of MHC II and iNOS (25, 33, 34). However, the underlying mechanism has not been studied in detail. In the present study, we investigated the impact of L. donovani amastigotes on IFN-γ responses and signaling via the JAK-STAT1 pathway. Our findings indicate that infection with L. donovani amastigotes causes the downregulation of IFN-γ-induced gene expression without affecting STAT1α activation. Rather, amastigotes inhibit IFN-γ-induced STAT1α nuclear translocation by blocking the interaction of STAT1α with the karyopherin importin-α5.
MATERIALS AND METHODS
Macrophage culture.
Bone marrow-derived macrophages (BMMs) were obtained from the femurs and tibias of 6- to 8-week-old female BALB/c mice (Charles River, St. Constant, QC, Canada) and differentiated as described previously (8, 27) in complete medium (Dulbecco's modified Eagle's medium with glutamine; Life Technologies, Burlington, ON, Canada) containing 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT), 20 mM HEPES, pH 7.4, and penicillin-streptomycin supplemented with 15% (vol/vol) L929 cell-conditioned medium, a source of colony-stimulating factor 1 (CSF-1), in a 37°C incubator with 5% CO2.
Parasites, infections, and IFN-γ stimulation.
Amastigotes of the Ethiopian LV9 strain of Leishmania donovani were isolated from the spleens of female HsdHan:AURA hamsters (Harlan Sprague Dawley Inc., Montreal, QC, Canada) that had been infected 8 to 12 weeks earlier with 1.5 × 108 amastigotes by intra-abdominal injection, as described previously (32). Promastigotes of L. donovani strain LV9, L. major NIH S clone A2, and L. amazonensis strain LV79 were cultured at 26°C in medium 199 supplemented with 10% heat-inactivated FBS, 100 μM adenine, 40 mM HEPES, 5 μM hemin, 3 μM biopterin, 1 μM biotin, and penicillin-streptomycin. Promastigotes were used in the late stationary phase of growth. For infections, BMMs were incubated at 37°C for either 18 h for amastigotes or 6 h for promastigotes at a parasite-to-macrophage ratio of 20:1, unless stated otherwise. Noninternalized parasites were removed by two washes in serum-free medium, and the cells were allowed to rest in serum-free medium for 1 h after each wash. Infection levels were assessed by microscopic examination of infected cells upon Giemsa staining. Over 90% of the BMMs were routinely infected with an average of 15 to 20 amastigotes per cell for a 20:1 ratio and 10 to 13 amastigotes per cell for a 10:1 ratio without affecting BMM viability. Recombinant mouse IFN-γ (R&D Systems, Minneapolis, MN) was added at 100 U/ml for the indicated times.
Reverse transcription-PCR (RT-PCR).
Total RNA was extracted from adherent BMMs using the TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA) and reverse transcribed as described previously (15). One-tenth of the resulting cDNA was amplified by PCR on a DNA thermal cycler, version 2.3 (Perkin-Elmer Corporation, Norwalk, CT), with the following primer pairs: for the α chain of MHC-II (I-Aα), 5′-GGA ATT CTG GGA ATC TCA GGT TCC CAG TG-3′ (forward) and 5′-GGA ATT CTG AAC ACC ATG CTC AGC CTC TG-3′ (reverse); for iNOS, 5′-CCG AAA CGC TTC ACT TCC AAT G-3′ (forward) and 5′-AAT CTC TGC CTA TCC GTC TCG TC-3′ (reverse); and for hypoxanthine phosphoribosyltransferase (HPRT), 5′-GTT GGA TAC AGG CCA GAC TTT GTT G-3′ (forward) and 5′-GAT TCA ACT TGC GCT CAT CTT AGG C-3′ (reverse). The PCR products were analyzed by electrophoresis on a 1.8% (wt/vol) agarose gel, and the pictures were taken using AlphaImager 3400 imaging software (Alpha Innotech Corporation, San Leandro, CA).
Quantitative real-time PCR.
Total RNA was extracted from adherent BMMs and reverse transcribed, as described above. One-tenth of the resulting cDNA was amplified by quantitative real-time PCR (qPCR) in a total volume of 20 μl, which contained 10 μl of PerfeCTa SYBR green SuperMix Low carboxy-X-rhodamine (ROX) reagent (Quanta Biosciences, Inc., Gaithersburg, MD), 7 μl of autoclaved high-pressure liquid chromatography-grade water (Fisher Scientific, Ottawa, ON, Canada), 2 μl of cDNA, and 500 nM the following forward and reverse primers: for I-Aα, 5′-TCA GTC GCA GAC GGT GTT TAT-3′ (forward) and 5′-GGG GGC TGG AAT CTC AGG T-3′ (reverse); for iNOS, 5′-CAG CAC AGG AAA TGT TTC AGC-3′ (forward) and 5′-TAG CCA GCG TAC CGG ATG A-3′ (reverse); and for HPRT, 5′-GTT GGA TAC AGG CCA GAC TTT GTT G-3′ (forward) and 5′-GAT TCA ACT TGC GCT CAT CTT AGG C-3′ (reverse). Amplifications were conducted in duplicate or triplicate on an MX3000P instrument under the following conditions: an initial melting step of 10 min at 95°C and 40 cycles of 95°C for 10 s, 60°C for 15 s, and 72°C for 20 s, with a final dissociation curve step being performed to ensure that there was only one (gene-specific) amplification peak for each primer pair. Cycle threshold (CT) values were recorded using MxPro-Mx3000P (version 3.20) Build 340 Schema 74 software and analyzed by the 2−ΔΔCT method.
Preparation of nuclear extracts and EMSA.
Adherent BMMs (107/dish) were washed once with ice-cold phosphate-buffered saline (PBS) and scraped into 1.5 ml of ice-cold PBS. Cell suspensions were transferred into microcentrifuge tubes and pelleted for 15 s at 4°C. Cytoplasm was removed by lysis in hypotonic buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT]) containing phosphatase inhibitors [1 mM Na3VO4, 50 mM NaF, 1.5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid [EGTA], 10 mM Na4P2O7] and complete protease inhibitors (Roche Applied Science, Laval, QC, Canada) for 10 min on ice, followed by addition of 0.5% Nonidet P-40 and vortexing for 10 s. Nuclei were pelleted by centrifugation for 15 s at 4°C and lysed in hypertonic buffer C (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 25% glycerol, and 0.5 mM DTT with phosphatase and protease inhibitors). After incubation on ice for 20 min, insoluble material was removed by centrifugation for 2 min at 4°C, and protein concentrations were determined using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). The electrophoretic mobility shift assay (EMSA) was performed essentially as described previously (15), using an oligonucleotide probe containing the consensus γ-activated sequence (GAS) recognized by STAT1 (5′-AGCCATTTCCAGGAATCGAAA-3′, where the underscore indicates the GAS). The gels were dried and exposed to an X-ray film at −70°C.
Immunoprecipitation and immunoblotting analysis.
For immunoblotting experiments, adherent BMMs (2 × 106/well) were washed with ice-cold PBS containing 1 mM Na3VO4 and lysed in 10 mM Tris-HCl, pH 7.5-1 mM EGTA-1% Triton X-100 with phosphatase and protease inhibitors. After incubation on ice for 10 min, total cell extracts were sonicated, insoluble material was removed by centrifugation for 10 min at 4°C, and protein concentrations were determined using the Pierce BCA protein assay. Western blotting was performed as described previously (39), using rabbit polyclonal antibodies against IRF1 (Santa Cruz Biotechnology, Santa Cruz, CA), Akt and pTyr701-STAT1 (Cell Signaling Technology, Beverly, MA), and the STAT1 C terminus and pSer727-STAT1 (Upstate Biotechnology, Lake Placid, NY). For immunoprecipitation analyses, adherent BMMs (107/dish) were washed with ice-cold PBS containing 1 mM Na3VO4 and lysed in 20 mM Tris-HCl, pH 7.5-10 mM EDTA, pH 8.0-1% Nonidet P-40 with phosphatase and protease inhibitors. After incubation on ice for 10 min, the lysates were sonicated and insoluble material was removed by centrifugation for 10 min at 4°C. The lysates were cleared for 1 h with recombinant protein G-Sepharose beads (Amersham Biosciences, Pittsburgh, PA) at 4°C. After removal of the protein G-Sepharose beads by centrifugation, the lysates were incubated for 2 h at 4°C with 1.5 μg of anti-importin-α5 antibody from Santa Cruz Biotechnology bound to protein G-Sepharose beads. Immunocomplexes were collected by brief centrifugation, washed three times with lysis buffer containing protease and phosphatase inhibitors, boiled in SDS sample buffer, and analyzed by Western blotting.
Confocal immunofluorescence microscopy.
BMMs were seeded in 24-well plates containing microscope coverslips (Fisher Scientific, Ottawa, ON, Canada) at 1.5 × 105/well, infected with L. donovani amastigotes for 18 h, and then stimulated with 100 U/ml IFN-γ for 15 min. The cells were washed in PBS, fixed with 2% paraformaldehyde for 10 min, and then simultaneously blocked and permeabilized in 0.1% Triton X-100-1% bovine serum albumin-20% normal goat serum-6% nonfat dry milk-50% FBS for 20 min at room temperature. The STAT1α distribution was visualized using a mouse anti-STAT1α (C terminus) IgG2b antibody from BD Transduction Laboratories at a 1:100 dilution and Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes, OR) at 1:500. Macrophage and amastigote nuclei were stained with DRAQ5 (BioStatus Ltd., United Kingdom) at 1:400. The coverslips were washed three times with PBS and mounted on the Fluoromount-G reagent (Interscience, Markham, ON, Canada). Analyses were performed using a Bio-Rad Radiance 2000 confocal imaging system (Bio-Rad Laboratories, Hercules, CA) installed on an Eclipse E800 microscope. STAT1α translocation was analyzed using an argon/krypton laser at 488 nm with a Plan Apo Nikon ×60 (numerical aperture [NA], 1.4) oil immersion lens. DRAQ5 fluorescence was analyzed using a 638-nm diode laser at 650-nm long pass with a Plan Apo Nikon ×60 (NA, 1.4) oil immersion lens. At least 50 cells from each of two independent experiments performed under each experimental condition were examined. Images were acquired in the normal scanning mode with a Kalman filter of 10 and LaserSharp software.
Densitometric analysis.
Band intensities were quantified by spot densitometry using AlphaImager 3400 imaging software (Alpha Innotech Corporation) and normalized to the value for the indicated control. Values are presented as the fold induction compared to the level in the uninfected, unstimulated sample.
Statistical analysis.
Data are presented as the means of three or more experiments ± standard deviation (SDs). The Student t test (two-tailed, unpaired) was performed in order to evaluate the significance of the differences observed between parasite-infected samples and their respective uninfected counterparts.
RESULTS
Downregulation of IFN-γ-induced gene expression by L. donovani amastigotes.
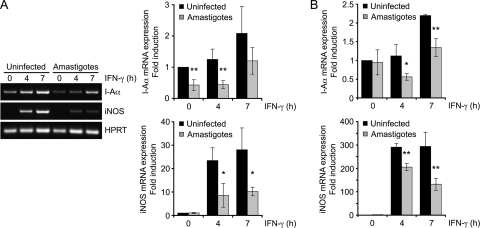
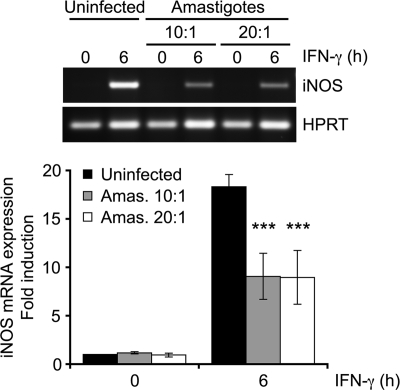
To study the mechanism by which L. donovani amastigotes inhibit IFN-γ-mediated responses, we first evaluated by RT-PCR (Fig. 1A) and RT-qPCR (Fig. 1B) the impact of L. donovani amastigotes on MHC II and iNOS gene expression in BMMs. Consistent with previous reports (33, 34), the IFN-γ-induced I-Aα mRNA levels were significantly reduced in BMMs infected for 18 h with L. donovani amastigotes compared to the levels in uninfected cells. Similarly, infection of BMMs with amastigotes significantly attenuated the IFN-γ-induced expression of iNOS. Downregulation of IFN-γ-induced iNOS expression remained highly significant when the parasite-to-cell ratio was lowered from 20:1 to 10:1 (Fig. 2). Altogether, these results are consistent with the notion that L. donovani amastigotes perturb the expression of the IFN-γ-inducible genes involved in microbe killing and antigen presentation.
FIG. 1.
L. donovani amastigotes downregulate IFN-γ-induced gene expression. Adherent BMMs were infected with freshly isolated L. donovani amastigotes for 18 h at a parasite-to-cell ratio of 20:1 and then stimulated with 100 U/ml IFN-γ for the indicated times. Total RNA was extracted, reverse transcribed, and analyzed by RT-PCR (A) or RT-qPCR (B) with primers specific for I-Aα, iNOS, and HPRT, as described in Materials and Methods. (A) Band intensities were quantified by densitometric analysis and normalized to those of the HPRT housekeeping gene; (B) the level of gene expression was normalized to that of HPRT and calculated via the 2−ΔΔCT method. Values are expressed as the fold difference from the results for the uninfected, unstimulated cells and represent the means ± SDs of three independent experiments (A) or of qPCR triplicates of an experiment representative of two others (B). *, P < 0.05; **, P < 0.02.
FIG. 2.
Impact of the infection ratio on the inhibition of IFN-γ-induced iNOS expression by L. donovani amastigotes. Adherent BMMs were infected with freshly isolated L. donovani amastigotes for 18 h at a parasite-to-cell ratio of 10:1 or 20:1 and then stimulated with 100 U/ml IFN-γ for the indicated times. Total RNA was extracted, reverse transcribed, and analyzed by RT-PCR with primers specific for iNOS and HPRT, as described in Materials and Methods. Band intensities were quantified by densitometric analysis and normalized to those for the HPRT housekeeping gene. Values are expressed as the fold difference of the results for the unstimulated, uninfected sample and represent the means ± SDs of two independent experiments performed with duplicate samples for each condition. ***, P < 0.001.
STAT1α levels and phosphorylation are normal in L. donovani amastigote-infected BMMs.
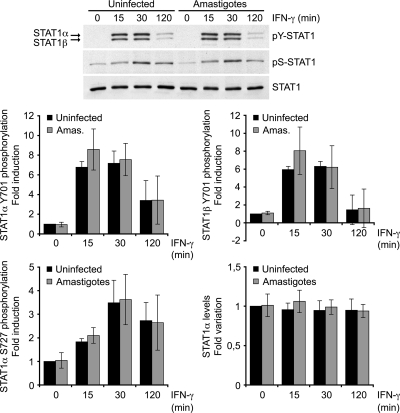
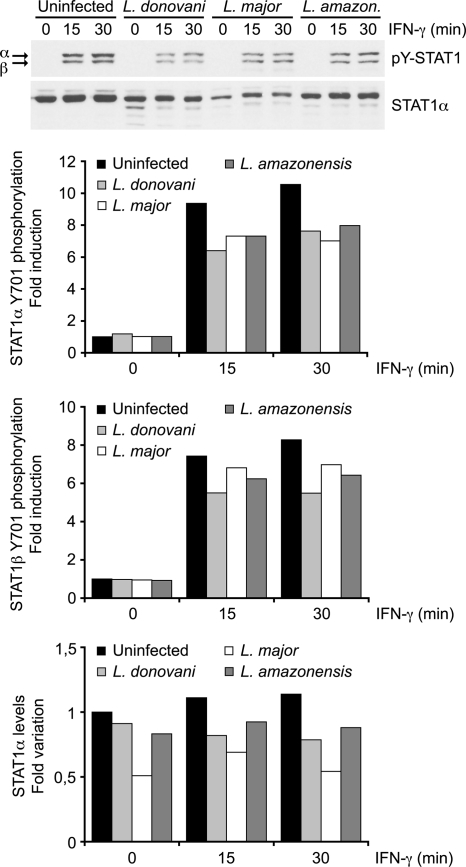
We next determined whether inhibition of IFN-γ-stimulated gene expression in L. donovani amastigote-infected BMMs resulted from a disruption of the JAK-STAT1 pathway. Western blot analyses revealed that STAT1α levels and the levels of IFN-γ-induced phosphorylation of STAT1α on both Tyr701 and Ser727 were similar in control and L. donovani amastigote-infected BMMs (Fig. 3). This was in contrast to previous reports indicating that Leishmania promastigotes inhibit IFN-γ signaling by preventing JAK2 and STAT1α activation and by inducing STAT1α degradation (4, 5, 11, 28). It was thus necessary to determine whether the inability of L. donovani amastigotes to impair STAT1α phosphorylation in BMMs was strain specific or developmental stage specific. To address this issue, we assessed the impact of promastigotes differentiated from our L. donovani LV9 amastigotes, as well as promastigotes from L. major and L. amazonensis, on STAT1α levels and IFN-γ-induced STAT1 phosphorylation. Consistent with previous reports (4, 5, 28), the level of STAT1 tyrosine phosphorylation was reduced in BMMs infected with promastigotes from L. donovani, L. major, and L. amazonensis compared to the level in uninfected BMMs (Fig. 4). Furthermore, as previously reported (11), STAT1α levels were also reduced in BMMs infected with either L. donovani or L. major promastigotes (Fig. 4). These results indicate that the mechanism by which L. donovani amastigotes inhibit IFN-γ signaling in BMMs is distinct from that used by promastigotes and occurs downstream of STAT1α activation.
FIG. 3.
L. donovani amastigotes do not alter protein levels or IFN-γ-induced phosphorylation of STAT1α. Adherent BMMs were infected with freshly isolated L. donovani amastigotes for 18 h at a parasite-to-cell ratio of 20:1 and then stimulated with 100 U/ml IFN-γ for the indicated times. Whole-cell lysates were separated by SDS-PAGE and analyzed by Western blotting using antibodies against pTyr701-STAT1, pSer727-STAT1α, the STAT1α C terminus, and Akt, as described in Materials and Methods. Densitometric analysis was performed and band intensities were normalized to those for the Akt control. Results are expressed as the fold difference from the results for the unstimulated, uninfected sample and represent the means ± SDs of at least three independent experiments.
FIG. 4.
Impact of Leishmania promastigotes from various species on STAT1α expression and activation. Adherent BMMs were infected with stationary-phase promastigotes of either L. donovani, L. major, or L. amazonensis for 6 h at a parasite-to-cell ratio of 20:1 and were then stimulated with 100 U/ml IFN-γ for the indicated times. Whole-cell lysates were separated by SDS-PAGE and analyzed by Western blotting using antibodies against pTyr701-STAT1 and the STAT1α C terminus, as described in Materials and Methods. Densitometric analysis was performed, and the results are expressed as the fold difference from the results for the unstimulated, uninfected sample. Data are representative of those from two independent experiments.
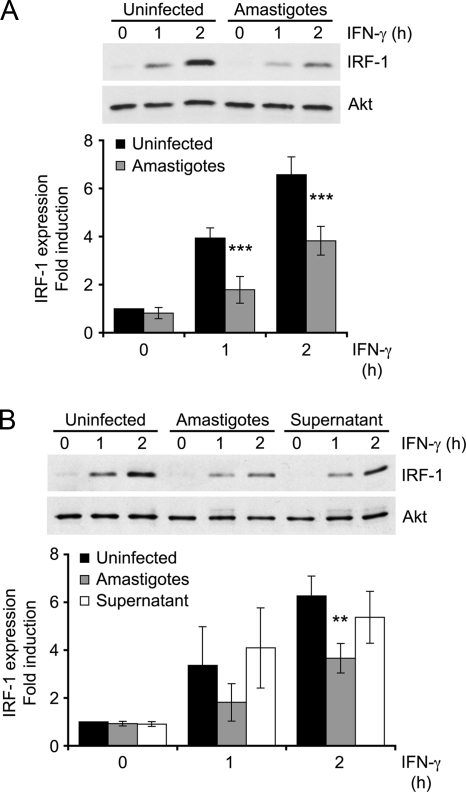
IFN-γ-induced IRF-1 expression is impaired by L. donovani amastigotes.
The transcription factor IRF-1 is among the first genes induced by STAT1α in response to IFN-γ, and they both act to regulate iNOS gene expression (18, 22, 24). We thus determined the impact of L. donovani amastigotes on IFN-γ-induced IRF-1 expression. In uninfected BMMs, IRF-1 was detectable by 1 h after IFN-γ stimulation and was stronger after 2 h (Fig. 5A). On the other hand, infection of BMMs with L. donovani amastigotes significantly impaired induction of IRF-1 expression by IFN-γ. Leishmania-infected macrophages release various immunomodulators which may impair IFN-γ responsiveness (26). To determine whether a secreted immunosuppressive molecule could be responsible for the inhibition of IFN-γ-induced IRF-1 expression in infected BMMs, we incubated uninfected BMMs with conditioned medium from macrophages infected with L. donovani amastigotes and analyzed IFN-γ-induced IRF-1 expression. Figure 5B shows that conditioned medium from infected BMMs had no impact on IRF-1 induction by IFN-γ, indicating that the mediators released by infected BMMs are not responsible for the unresponsiveness to IFN-γ.
FIG. 5.
Inhibition of IFN-γ-induced IRF-1 expression by L. donovani amastigotes. Adherent BMMs were either infected with freshly isolated L. donovani amastigotes for 18 h at a parasite-to-cell ratio of 20:1 (A and B) or incubated for 5 h with the supernatant of infected BMMs (B). The cells were then stimulated with 100 U/ml IFN-γ for the indicated times. Total cell extracts were analyzed by Western blotting with antibodies against IRF-1 and Akt, as described in Materials and Methods. Densitometric analysis was performed, and IRF-1 band intensities were normalized to those for the Akt control. Results are expressed as the fold difference from the results for the unstimulated, uninfected sample and represent the means ± SDs of five (A) or four (B) independent experiments. **, P < 0.02; ***, P < 0.001.
L. donovani amastigotes prevent IFN-γ-induced STAT1α nuclear translocation by blocking STAT1α association with importin-α5.
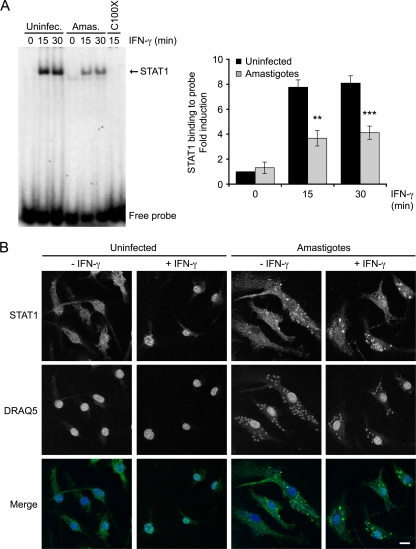
The finding that IRF-1 expression was defective in infected BMMs led us to verify the possibility that L. donovani amastigotes tamper with the ability of activated STAT1α to translocate to the nucleus and/or to bind to DNA. We first assessed by EMSA the levels of active STAT1α present in nuclear extracts from uninfected and amastigote-infected BMMs stimulated with IFN-γ for 15 and 30 min. As shown in Fig. 6A, the level of binding of the STAT1α present in nuclear extracts to a GAS-containing oligonucleotide probe was significantly reduced in L. donovani amastigote-infected BMMs compared to that observed in uninfected cells. We next examined by confocal immunofluorescence microscopy the impact of an amastigote infection on the intracellular redistribution of STAT1α upon treatment with IFN-γ for 15 min. As shown in Fig. 6B, the massive nuclear translocation of STAT1α observed in IFN-γ-stimulated uninfected BMMs was strongly attenuated in infected cells, confirming that L. donovani amastigotes interfere with IFN-γ-induced STAT1α nuclear translocation.
FIG. 6.
L. donovani amastigotes inhibit the nuclear translocation of STAT1α induced by IFN-γ. BMMs were infected with freshly isolated L. donovani amastigotes for 18 h at a parasite-to-cell ratio of 20:1 and then stimulated with 100 U/ml IFN-γ for the indicated time points. (A) Nuclear extracts were analyzed by EMSA for the level of STAT1 binding to a GAS consensus oligonucleotide probe. Cold probe (C100X) was added at 100 times the concentration of radioactive probe to the reaction mixture of the uninfected sample stimulated with IFN-γ for 15 min as a competition to verify the specificity of the bands. Band intensities were quantified by spot densitometry. Values are expressed as the fold difference from the results for the unstimulated, uninfected sample and represent the means ± SDs of three independent experiments. (B) After incubation for 18 h in the presence or absence of amastigotes and subsequent treatment with IFN-γ for 15 min, the cells were fixed and stained with the DNA marker DRAQ5 (blue) and with the anti-STAT1α C terminus antibody (green) for immunofluorescence confocal microscopy, as described in Materials and Methods. Scale bar, 3 μm. Results are representative of two independent experiments. **, P < 0.02; ***, P < 0.001.
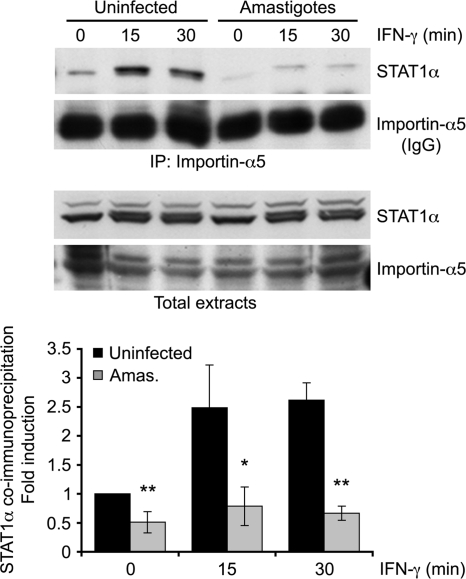
Transport of STAT1α from the cytoplasm to the nucleus requires the nuclear transport adaptor importin-α5 (23, 37). We thus determined whether a defective association between STAT1α and importin-α5 could account for the inhibition of IFN-γ-induced STAT1α nuclear translocation by L. donovani amastigotes. To this end, we immunoprecipitated importin-α5 from uninfected and L. donovani amastigote-infected BMMs 15 and 30 min after the addition of IFN-γ and looked for the presence of STAT1α in the immunoprecipitates. As shown in Fig. 7, IFN-γ rapidly increased the levels of STAT1α present in importin-α5 immunoprecipitates from uninfected BMMs. In contrast, the levels of STAT1α present in importin-α5 immunoprecipitates from L. donovani amastigote-infected BMMs were drastically reduced, both before and after the addition of IFN-γ. These results establish that L. donovani amastigotes inhibit the association between STAT1α and importin-α5, thereby preventing the nuclear translocation of STAT1α.
FIG. 7.
L. donovani amastigotes block STAT1α association with importin-α5 in response to IFN-γ. Adherent BMMs were infected with freshly isolated L. donovani amastigotes for 18 h at a parasite-to-cell ratio of 20:1 and then stimulated with 100 U/ml IFN-γ for the indicated times. Total cell extracts were immunoprecipitated using an anti-importin-α5 antibody, as described in Materials and Methods. Immune complexes and whole-cell extracts (input) were subjected to Western blot analysis using antibodies against the STAT1α C terminus and importin-α5. As immunoprecipitated importin-α5 is masked by the anti-importin-α5 antibody heavy chain (IgG), the band intensities for immunoprecipitated STAT1α were normalized to the intensity for a nonspecific band. Values are expressed as the fold difference from the results for the unstimulated, uninfected sample and represent the means ± SDs of three independent experiments. *, P < 0.05; **, P < 0.02.
DISCUSSION
The present study was aimed at investigating how L. donovani amastigotes interfere with the triggering of IFN-γ-inducible macrophage functions. We report that in BMMs, L. donovani amastigotes impair the nuclear translocation of STAT1α by preventing its association with the importin-α5 shuttling receptor.
Alteration of macrophage signaling regulatory machineries is one of the strategies used by the parasite Leishmania to avoid activation of microbicidal mechanisms and detection by the host immune system (19, 26). Previous studies revealed that Leishmania promastigotes use diverse stratagems to efficiently target the JAK2-STAT1 signaling cascade in order to attenuate IFN-γ-inducible macrophage functions (4, 5, 11, 28). These include activation of host cell tyrosine phosphatases, inactivation of JAK2, and massive tyrosine dephosphorylation of macrophage proteins, as well as proteasome-mediated degradation of STAT1α. Recent evidence revealed that alteration of JAK-STAT signaling and modulation of protein tyrosine phosphatase activity by promastigotes involve the proteolytic activity of the promastigote surface protease GP63, which appears to access the host cell cytoplasm by a lipid raft-dependent mechanism (16).
In contrast, little is known of the mechanisms by which amastigotes prevent macrophage activation by IFN-γ. This knowledge is crucial for the development of novel prophylactic and therapeutic approaches against leishmaniases, as the amastigote stage is responsible for disease progression and pathology. As previously reported (33, 34), we found that L. donovani amastigotes strongly reduce IFN-γ-induced MHC II expression at the level of gene transcription. L. donovani amastigotes have also been reported to antagonize induction of iNOS protein expression by IFN-γ in macrophage cell lines (25). Consistently, we observed a downregulation of iNOS gene expression in L. donovani amastigote-infected BMMs. Expression of iNOS in IFN-γ-activated macrophages is dependent on the transcription factors STAT1α and IRF-1 (18, 22, 24). This led us to verify the impact of L. donovani amastigotes on these two key mediators of IFN-γ responses. We found that L. donovani amastigotes neither altered STAT1α protein levels nor prevented STAT1α Tyr701 and Ser727 phosphorylation. Normal STAT1α activation indicates that the upstream steps in the IFN-γ signaling cascade are not affected in L. donovani amastigote-infected BMMs. It is thus unlikely that impaired IFN-γ-induced gene expression is related to the downregulation of the IFN-γ receptor (4, 28). On the other hand, induction of IRF-1 expression was defective in L. donovani amastigote-infected BMMs. Since IFN-γ-induced IRF-1 expression requires STAT1α activation, we reasoned that the downregulation of IRF-1 expression in infected BMMs may be the consequence of an inability of STAT1α to either translocate to the nucleus or bind to DNA. Indeed, our findings indicate that L. donovani amastigotes hinder STAT1α translocation to the nucleus, by abrogating IFN-γ-induced STAT1α interaction with the nuclear transport adaptor importin-α5. This mechanism is distinct from the recently described mechanism by which L. mexicana amastigotes prevent nuclear translocation of various transcription factors, including STAT1, through proteolytic degradation involving the cysteine proteinase LmCPb (1).
Previous studies showed that as they initiate infection, Leishmania promastigotes activate host cell protein tyrosine phosphatases to efficiently shut off IFN-γ-activated macrophage microbicidal mechanisms (4, 5, 11, 28, 43). However, given their relatively generalized effect on cellular functions, sustained activation of protein tyrosine phosphatases such as SHP-1 would be detrimental to macrophage survival. We found that infection of BMMs with L. donovani amastigotes was not accompanied by significant changes in tyrosine phosphorylation levels (data not shown). This is consistent with the notion that L. donovani amastigotes act at a very specific step of the JAK-STAT1 signaling cascade, which nevertheless results in an efficient attenuation of IFN-γ-induced MHC II and iNOS expression. In this way, amastigotes may replicate in IFN-γ-unresponsive macrophages without compromising the survival of these cells.
The mechanism by which L. donovani amastigotes disrupt the STAT1α-importin-α5 association remains to be elucidated. One attractive hypothesis is that a parasite-derived molecule binds to and sequesters away activated STAT1α or importin-α5 or acts on a pathway that regulates the interaction between STAT1α and importin-α5. Analogous mechanisms have been described for viruses (13, 30, 31). However, the seclusion of Leishmania amastigotes within a parasitophorous vacuole supposes the existence of a mechanism allowing a putative parasite effector to cross the vacuole membrane and to thwart the association of STAT1α with importin-α5. The recently described Leishmania exosome-based secretion mechanism may play a role in this process (38). Alternatively, the elevated ceramide levels observed in L. donovani-infected macrophages (14) may interfere with the nuclear import of importin-α5 (10). Clearly, disruption of the association between STAT1α and importin-α5 by L. donovani amastigotes provides a unique system with which to study the regulation of this association, for which little is currently known.
While the biological implications of Ser727 phosphorylation on STAT1α transcriptional activity are well characterized (7, 41, 42), the underlying mechanism of Ser727 phosphorylation in response to IFN-γ is still poorly understood. Recent studies using immortalized mouse embryonic fibroblasts showed that IFN-γ-induced STAT1α Ser727 phosphorylation requires prior Tyr701 phosphorylation, nuclear translocation, and a stable association of STAT1α with chromatin (35). Our observation that L. donovani amastigotes interfere with STAT1α nuclear import without affecting Ser727 phosphorylation suggests that this phosphorylation event occurs in the cytoplasm in macrophages and raises the hypothesis that distinct mechanisms may regulate STAT1α Ser727 phosphorylation in response to IFN-γ in these two cell types.
In conclusion, this study brings to light a novel mechanism exploited by Leishmania parasites to disrupt a key signaling cascade associated with macrophage activation, whereby the amastigote stage prevents the nuclear translocation of STAT1α in response to IFN-γ by blocking its interaction with importin-α5. Identification of the host and parasite components involved in this inhibition will be crucial to our understanding of Leishmania pathogenesis. As Toxoplasma gondii was reported to inhibit STAT1 nuclear translocation (21), future studies may reveal whether disruption of the STAT1α-importin-α5 interaction is a strategy used by other intracellular protozoan parasites to prevent STAT1α-mediated gene expression.
Acknowledgments
This work was supported by Canadian Institutes of Health Research grant MOP-12933. C.M. was supported by studentships from the Canadian Institutes of Health Research and the Fonds de la Recherche en Santé du Québec. A.D. is the holder of a Canada research chair and was a chercheur-boursier from the FRSQ. The Center for Host-Parasite Interactions is supported by the Fonds Québécois de la Recherche sur la Nature et les Technologies.
We are grateful to Marcel Desrosiers and Adrien Vinet for their help with confocal immunofluorescence microscopy.
Editor: J. H. Adams
Footnotes
Published ahead of print on 21 June 2010.
REFERENCES
- 1.Abu-Dayyeh, I., K. Hassani, E. R. Westra, J. C. Mottram, and M. Olivier. 5 April 2010. Comparative study of the ability of Leishmania mexicana promastigotes and amastigotes to alter macrophage signalling and functions. Infect. Immun. doi: 10.1128/IAI.00812-09. [DOI] [PMC free article] [PubMed]
- 2.Ben-Othman, R., L. Guizani-Tabbane, and K. Dellagi. 2008. Leishmania initially activates but subsequently down-regulates intracellular mitogen-activated protein kinases and nuclear factor-kappaB signaling in macrophages. Mol. Immunol. 45:3222-3229. [DOI] [PubMed] [Google Scholar]
- 3.Bertholet, S., H. L. Dickensheets, F. Sheikh, A. A. Gam, R. P. Donnelly, and R. T. Kenney. 2003. Leishmania donovani-induced expression of suppressor of cytokine signaling 3 in human macrophages: a novel mechanism for intracellular parasite suppression of activation. Infect. Immun. 71:2095-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj, N., L. E. Rosas, W. P. Lafuse, and A. R. Satoskar. 2005. Leishmania inhibits STAT1-mediated IFN-gamma signaling in macrophages: increased tyrosine phosphorylation of dominant negative STAT1beta by Leishmania mexicana. Int. J. Parasitol. 35:75-82. [DOI] [PubMed] [Google Scholar]
- 5.Blanchette, J., N. Racette, R. Faure, K. A. Siminovitch, and M. Olivier. 1999. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-gamma-triggered JAK2 activation. Eur. J. Immunol. 29:3737-3744. [DOI] [PubMed] [Google Scholar]
- 6.Boss, J. M. 1997. Regulation of transcription of MHC class II genes. Curr. Opin. Immunol. 9:107-113. [DOI] [PubMed] [Google Scholar]
- 7.Decker, T., and P. Kovarik. 2000. Serine phosphorylation of STATs. Oncogene 19:2628-2637. [DOI] [PubMed] [Google Scholar]
- 8.Descoteaux, A., and G. Matlashewski. 1989. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol. Cell. Biol. 9:5223-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dogra, N., C. Warburton, and W. R. McMaster. 2007. Leishmania major abrogates gamma interferon-induced gene expression in human macrophages from a global perspective. Infect. Immun. 75:3506-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faustino, R. S., P. Cheung, M. N. Richard, E. Dibrov, A. L. Kneesch, J. F. Deniset, M. N. Chahine, K. Lee, D. Blackwood, and G. N. Pierce. 2008. Ceramide regulation of nuclear protein import. J. Lipid Res. 49:654-662. [DOI] [PubMed] [Google Scholar]
- 11.Forget, G., D. J. Gregory, and M. Olivier. 2005. Proteasome-mediated degradation of STAT1alpha following infection of macrophages with Leishmania donovani. J. Biol. Chem. 280:30542-30549. [DOI] [PubMed] [Google Scholar]
- 12.Forget, G., D. J. Gregory, L. A. Whitcombe, and M. Olivier. 2006. Role of host protein tyrosine phosphatase SHP-1 in Leishmania donovani-induced inhibition of nitric oxide production. Infect. Immun. 74:6272-6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frieman, M., B. Yount, M. Heise, S. A. Kopecky-Bromberg, P. Palese, and R. S. Baric. 2007. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 81:9812-9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh, S., S. Bhattacharyya, S. Das, S. Raha, N. Maulik, D. K. Das, S. Roy, and S. Majumdar. 2001. Generation of ceramide in murine macrophages infected with Leishmania donovani alters macrophage signaling events and aids intracellular parasitic survival. Mol. Cell. Biochem. 223:47-60. [DOI] [PubMed] [Google Scholar]
- 15.Giroux, M., M. Schmidt, and A. Descoteaux. 2003. IFN-gamma-induced MHC class II expression: transactivation of class II transactivator promoter IV by IFN regulatory factor-1 is regulated by protein kinase C-alpha. J. Immunol. 171:4187-4194. [DOI] [PubMed] [Google Scholar]
- 16.Gomez, M. A., I. Contreras, M. Halle, M. L. Tremblay, R. W. McMaster, and M. Olivier. 2009. Leishmania GP63 alters host signaling through cleavage-activated protein tyrosine phosphatases. Sci. Signal 2:ra58. [DOI] [PubMed] [Google Scholar]
- 17.Jayakumar, A., R. Widenmaier, X. Ma, and M. A. McDowell. 2008. Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages. J. Parasitol. 94:84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamijo, R., H. Harada, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, S. J. Green, et al. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 19.Kima, P. E. 2007. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 37:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodge, R., T. O. Diallo, and A. Descoteaux. 2006. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell. Microbiol. 8:1922-1931. [DOI] [PubMed] [Google Scholar]
- 21.Lüder, C. G., W. Walter, B. Beuerle, M. J. Maeurer, and U. Gross. 2001. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1alpha. Eur. J. Immunol. 31:1475-1484. [DOI] [PubMed] [Google Scholar]
- 22.Martin, E., C. Nathan, and Q. W. Xie. 1994. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 180:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride, K. M., G. Banninger, C. McDonald, and N. C. Reich. 2002. Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-alpha. EMBO J. 21:1754-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meraz, M. A., J. M. White, K. C. Sheehan, E. A. Bach, S. J. Rodig, A. S. Dighe, D. H. Kaplan, J. K. Riley, A. C. Greenlund, D. Campbell, K. Carver-Moore, R. N. DuBois, R. Clark, M. Aguet, and R. D. Schreiber. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431-442. [DOI] [PubMed] [Google Scholar]
- 25.Nandan, D., R. Lo, and N. E. Reiner. 1999. Activation of phosphotyrosine phosphatase activity attenuates mitogen-activated protein kinase signaling and inhibits c-FOS and nitric oxide synthase expression in macrophages infected with Leishmania donovani. Infect. Immun. 67:4055-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivier, M., D. J. Gregory, and G. Forget. 2005. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 18:293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Privé, C., and A. Descoteaux. 2000. Leishmania donovani promastigotes evade the activation of mitogen-activated protein kinases p38, c-Jun N-terminal kinase, and extracellular signal-regulated kinase-1/2 during infection of naive macrophages. Eur. J. Immunol. 30:2235-2244. [DOI] [PubMed] [Google Scholar]
- 28.Ray, M., A. A. Gam, R. A. Boykins, and R. T. Kenney. 2000. Inhibition of interferon-gamma signaling by Leishmania donovani. J. Infect. Dis. 181:1121-1128. [DOI] [PubMed] [Google Scholar]
- 29.Reich, N. C., and L. Liu. 2006. Tracking STAT nuclear traffic. Nat. Rev. Immunol. 6:602-612. [DOI] [PubMed] [Google Scholar]
- 30.Reid, S. P., L. W. Leung, A. L. Hartman, O. Martinez, M. L. Shaw, C. Carbonnelle, V. E. Volchkov, S. T. Nichol, and C. F. Basler. 2006. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 80:5156-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid, S. P., C. Valmas, O. Martinez, F. M. Sanchez, and C. F. Basler. 2007. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J. Virol. 81:13469-13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiner, N. E. 1982. Host-parasite relationship in murine leishmaniasis: pathophysiological and immunological changes. Infect. Immun. 38:1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiner, N. E., W. Ng, T. Ma, and W. R. McMaster. 1988. Kinetics of gamma interferon binding and induction of major histocompatibility complex class II mRNA in Leishmania-infected macrophages. Proc. Natl. Acad. Sci. U. S. A. 85:4330-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiner, N. E., W. Ng, and W. R. McMaster. 1987. Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J. Immunol. 138:1926-1932. [PubMed] [Google Scholar]
- 35.Sadzak, I., M. Schiff, I. Gattermeier, R. Glinitzer, I. Sauer, A. Saalmuller, E. Yang, B. Schaljo, and P. Kovarik. 2008. Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. Proc. Natl. Acad. Sci. U. S. A. 105:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 37.Sekimoto, T., N. Imamoto, K. Nakajima, T. Hirano, and Y. Yoneda. 1997. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 16:7067-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman, J. M., J. Clos, C. C. de'Oliveira, O. Shirvani, Y. Fang, C. Wang, L. J. Foster, and N. E. Reiner. 2010. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 123:842-852. [DOI] [PubMed] [Google Scholar]
- 39.St-Denis, A., F. Chano, P. Tremblay, Y. St-Pierre, and A. Descoteaux. 1998. Protein kinase C-alpha modulates lipopolysaccharide-induced functions in a murine macrophage cell line. J. Biol. Chem. 273:32787-32792. [DOI] [PubMed] [Google Scholar]
- 40.Steimle, V., C. A. Siegrist, A. Mottet, B. Lisowska-Grospierre, and B. Mach. 1994. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 265:106-109. [DOI] [PubMed] [Google Scholar]
- 41.Varinou, L., K. Ramsauer, M. Karaghiosoff, T. Kolbe, K. Pfeffer, M. Muller, and T. Decker. 2003. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity 19:793-802. [DOI] [PubMed] [Google Scholar]
- 42.Wen, Z., Z. Zhong, and J. E. Darnell, Jr. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241-250. [DOI] [PubMed] [Google Scholar]
- 43.Xin, L., K. Li, and L. Soong. 2008. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol. Immunol. 45:3371-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]