Abstract
Enteroaggregative Escherichia coli (EAEC) strains are important diarrheal pathogens. EAEC strains are defined by their characteristic stacked-brick pattern of adherence to epithelial cells but show heterogeneous virulence and have different combinations of adhesin and toxin genes. Pathoadaptive deletions in the lysine decarboxylase (cad) genes have been noted among hypervirulent E. coli subtypes of Shigella and enterohemorrhagic E. coli. To test the hypothesis that cad deletions might account for heterogeneity in EAEC virulence, we developed a Caenorhabditis elegans pathogenesis model. Well-characterized EAEC strains were shown to colonize and kill C. elegans, and differences in virulence could be measured quantitatively. Of 49 EAEC strains screened for lysine decarboxylase activity, 3 tested negative. Most notable is isolate 101-1, which was recovered in Japan, from the largest documented EAEC outbreak. EAEC strain 101-1 was unable to decarboxylate lysine in vitro due to deletions in cadA and cadC, which, respectively, encode lysine decarboxylase and a transcriptional activator of the cadAB genes. Strain 101-1 was significantly more lethal to C. elegans than control strain OP50. Lethality was attenuated when the lysine decarboxylase defect was complemented from a multicopy plasmid and in single copy. In addition, restoring lysine decarboxylase function produced derivatives of 101-1 deficient in aggregative adherence to cultured human epithelial cells. Lysine decarboxylase inactivation is pathoadapative in an important EAEC outbreak strain, and deletion of cad genes could produce hypervirulent EAEC lineages in the future. These results suggest that loss, as well as gain, of genetic material can account for heterogeneous virulence among EAEC strains.
Enteroaggregative Escherichia coli (EAEC) strains are etiologic agents of diarrhea. Bhan et al. (5) initially defined a role for EAEC in persistent diarrhea in rural Indian children. Since then, EAEC strains have been implicated in sporadic acute and persistent diarrhea in industrialized as well as developing countries (reviewed in references 24, 27, 28, and 46). EAEC isolates have also been implicated in a number of diarrheal disease outbreaks (12, 23, 29, 48, 55). EAEC strains adhere to the intestinal epithelium in a signature stacked-brick fashion. They form a copious biofilm and deliver one or more toxins to the host to precipitate diarrhea. Toxins include the enteroaggregative heat-stable toxin, a plasmid-encoded enterotoxin (Pet), and Shigella enterotoxins 1 and 2 (reviewed in references 24 and 46). EAEC strains are exceptional colonizers; many EAEC strains carry at least one of four types of aggregative adherence fimbriae (AAF) and may also possess afimbrial adhesins (4, 6, 8, 11, 15, 17, 39, 42). In addition, several EAEC strains express dispersin, an antiaggregation protein (Aap), believed to promote detachment from EAEC biofilms and seeding of new infection foci (54). Heterogeneity is an established feature of EAEC; no known EAEC strain produces all of the known EAEC virulence factors.
Gene acquisition is an important evolutionary route to virulence, but pathogenicity can also be enhanced by the loss of genetic material. For example, pathoadaptive deletions, or “black holes,” in Shigella genomes are the basis for a biochemical deficiency that also enhances virulence (16, 34). The E. coli cad operon contributes to acid resistance through the product of the cadA gene, lysine decarboxylase. Lysine decarboxylase converts lysine to the basic polyamine cadaverine, which is exported from the cell by the lysine-cadaverine antiporter CadB. The cadAB genes are activated under conditions of low pH and/or high lysine by CadC (37, 38).
Deletion of the cad genes in Shigella appears to have occurred as separate events in different Shigella lineages, suggestive of negative selection against cadABC in that pathotype (16). Maurelli et al. (34) reported that cad deletion enhances virulence because cadaverine, the product of lysine decarboxylation, inhibits the activity of Shigella enterotoxin 1 (34). More recently, it has been demonstrated that pathoadaptive cadAB deletions occur in E. coli lineages other than Shigella and enteroinvasive E. coli. These include lineages of Shiga toxin-producing E. coli (including enterohemorrhagic E. coli, EHEC), enteropathogenic E. coli, and enterotoxigenic E. coli (31, 63, 65). These pathotypes do not have Shigella enterotoxin 1 genes. Instead, restoration of lysine decarboxylase activity reduces adherence in naturally occurring cad mutants (63, 65). In the case of EHEC, the transcription of multiple adhesins is upregulated in a cadA deletion mutant, compared to the isogenic wild-type strain (65).
Although the EAEC pathotype is heterogeneous (14, 46), pathoadaptive deletions have not been previously reported. EAEC strains are hyperadherent, and, unlike many other E. coli pathotypes, a number of them possess the Shigella enterotoxin 1 genes (set1AB) (14, 46). As deletion of cadAB has occurred in multiple enteric lineages, we hypothesized that at least some EAEC strains might harbor this deletion and that it could account for some of the heterogeneity in virulence seen among EAEC strains. Since there is no model of EAEC infection that measures virulence in association with colonization, we developed a Caenorhabditis elegans model for EAEC and used it to assess whether any cadAB deletions were pathoadaptive for EAEC.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Forty-nine previously studied test strains were used, including 26 representatives from the major EAEC lineages identified by multilocus enzyme electrophoresis (MLEE) and 15 strains from a Nigerian case control study (30, 45) (see Table S1 in the supplemental material). Shigella flexneri 2a strain 2425T, enteroinvasive E. coli strain EI 37, and E. coli K-12 strain MG1655 were used as controls. E. coli K-12 strains TOP10 and DH5α (Invitrogen) were used as hosts for cloned genes. E. coli OP50 (32) was used for routine C. elegans feeding and as a negative control in slow-kill experiments, while Pseudomonas aeruginosa PA14 was a positive control. Bacteria were cultured in Luria broth (LB) or LB agar. Ampicillin (100 μg/ml routinely, 20 μg/ml in C. elegans plates), chloramphenicol (30 μg/ml), tetracycline (25 μg/ml), or neomycin (50 μg/ml) was added for selection when required. Strains were maintained in LB-glycerol (1:1) at −80°C. Plasmids used in this study are listed in Table 1.
TABLE 1.
Plasmids and constructs used in this study
| Plasmid or construct | Description | Antimicrobial resistance marker(s)a | Reference or source |
|---|---|---|---|
| pGEMT | TA cloning vector | Amp | Promega |
| pCADA | cadA plasmid clone under the control of the lac promoter | Km | 34, 38 |
| pUC4K | Kanamycin resistance gene from Tn903 in pUC18 | Amp Km | 58 |
| pGFP | GFP-encoding ampicillin-resistant plasmid | Amp | Clontech |
| pHTK4 | dsRed-encoding trimethoprim-resistant plasmid | Tmp | 61 |
| pGRG36 | Temperature-sensitive attTn7 delivery vector | Amp | 35 |
| pGRG36K | Kanamycin resistance gene from pUC4K, excised with HincII | Amp Km | This study |
| pINK1300 | cadABC with 380-bp upstream sequence from E. coli MG1655 in the TA cloning site of pGEMT | Amp | This study |
| pINK1307 | NotI insert from pINK1300 cloned into the SmaI site of pGRG36K | Amp Km | This study |
| INK1300 | EAEC strain 101-1 with cadABC chromosomally integrated at attTn7 | Amp Km Cm | This study |
Amp, ampicillin; Km, kanamycin/neomycin; Cm, chloramphenicol; Tmp, trimethoprim.
Routine molecular biology procedures.
Standard molecular biology procedures were employed (53). Plasmids were electroporated into EAEC strains using a Bio-Rad Micropulser, according to the manufacturer's instructions. Test EAEC strains were screened by PCR for lysine decarboxylase genes cadA, cadAB, and cadC, as well as a region immediately downstream of cadC, spanning from yjdC to dsbD (dipZ), which we amplified for control purposes. We also screened the strains for set1A, encoding the toxigenic subunit of Shigella enterotoxin 1. Oligonucleotides employed to amplify each locus are listed in Table S1 in the supplemental material. cadAB was amplified with Taq Long Plus polymerase (Stratagene) in accordance with manufacturers’ instructions and annealing at 42°C. All other DNA amplifications were performed using 1 unit of recombinant Taq polymerase enzyme (Invitrogen), 2 mM MgCl2, PCR buffer (Invitrogen), and 1 μM oligonucleotide primer in each reaction. All amplifications began with a 2-min hot start at 94°C followed by 30 cycles of denaturing at 94°C for 30 s, annealing for 30 s at 5°C below primer annealing temperature, and extending at 72°C for 1 min for every kilobase of amplified DNA. Genomic DNA served as the template.
Construction of a 101-1 derivative carrying cadABC in single copy on the chromosome.
A derivative of the Tn7-based transposition vector pGRG36 (35) was used to site-specifically integrate the cadABC genes and a kanamycin/neomycin resistance marker into attTn7 on the chromosome of 101-1. Temperature-sensitive plasmid pGRG36 and its derivatives were maintained at 30°C. A kanamycin- and neomycin-resistant vector derivative, pGRG36K, was prepared by cloning a 1,252-bp aminoglycoside phosphotransferase gene cassette (originally from Tn903) excised with HincII from pUC4K (58) into the SmaI site of pGRG36. Primers cadCpromF and cadAR were used to amplify the cadCBA genes and 380 bp upstream of cadC from E. coli K-12 strain MG1655. The 5.8-kb product was amplified using Long and Accurate (LA) Taq polymerase (Takara) according to the manufacturer's instructions, annealing at 50°C for 1 60 s, and extending at 68°C for 6 min, and then cloned into the TA cloning vector pGEMT-Easy (Promega). The resulting clone, pINK1300, was confirmed by end sequencing and by its ability to confer lysine decarboxylase activity on 101-1. The cadCBA insert was subcloned into the NotI site of pGRG36K to produce pINK1307. Both the neomycin/kanamycin resistance cassette and the cad genes lie adjacent to one another and between the Tn7 recognition sites of pINK1307. Integration of these genes at attTn7 (between pstS and glmS) on the chromosome of 101-1 was effected as described by McKenzie and Craig (35). Briefly, plasmid pINK1307 was electroporated into 101-1 and transformants were selected on LB containing neomycin and ampicillin at 30°C. Transformants were cultured overnight without selection in Luria broth at room temperature, tnsABCD gene transcription was induced from an arabinose promoter for 2 h by addition of 0.2% arabinose, and dilutions of the induced culture were plated on LB with neomycin and incubated at 42°C overnight. Following one subculture at 42°C overnight, 101-1::cadABC candidates were identified by screening for lysine decarboxylase activity. The genotype of positive candidates was verified by PCR with primer pairs cadCF/cadCR, pstSF/glmSR, and cadCR/pstSF. We also verified that, other than acquisition of neomycin resistance, strain INK1300, the cadABC-positive derivative of 101-1, had a resistance profile, growth characteristics, colony morphology on selective-diagnostic media, and a plasmid profile identical to those of the wild type.
Lysine decarboxylase test.
Lysine decarboxylase activity was determined using commercially available lysine decarboxylase broth (Difco) according to the method described by Falkow (19). E. coli MG1655 and S. flexneri 2a 2425T were used as positive and negative controls, respectively.
C. elegans slow-kill assay.
The standard wild-type N2 Bristol strain was used for all assays, and the slow-kill assay protocol was adapted from the work of Tan et al. (57). Worms were maintained on nematode growth medium (NGM) (9, 32) on lawns of E. coli OP50 at 15°C. Bacterial strains used in the assay are listed in Table 2. Test plates were prepared by spreading 5 μl of overnight bacterial culture on 3.5-cm-diameter plates containing NGM. Where applicable, cadaverine (Sigma) was incorporated into the medium at 300 μM. All plates were incubated at 37°C for 12 to 24 h and then placed at room temperature for 8 to 12 h. For the slow-kill assay, 10 N2 hermaphrodites at the L4 stage were seeded onto each plate, which was kept at room temperature for the remainder of the assay. Worms were transferred to new inoculated plates every other day to allow the distinction of subsequent generations. Survival was assayed every 12 or every 24 h, and worms were considered dead when they no longer responded to touch. Strains PA14 and OP50 were used as positive and negative controls, respectively, and each independent assay was repeated in quadruple. Data from the assays were analyzed using the Kaplan-Meier method (7) using the PEPI Version 4.0 SURVIVAL program.
TABLE 2.
Wild-type bacteria fed to C. elegans in survival assays
| Strain | MLEE-derived phylogenetic groupa | Known EAEC virulence genesb |
|---|---|---|
| OP50 | NDc | ND |
| P. aeruginosa PA14 | Not applicable | ND |
| 60A | EAEC1 | aagA aggR aap irp2 set1 pic |
| 17-2 | EAEC1 | shf aagA aggR aap irp2 |
| 101-1 | None (not EAEC1, EAEC2, or AA/DA) | shf aafA aap |
| 042 | EAEC2 | shf aafA pet aggR aap chuA irp2 set1 pic |
| H145-1 | EAEC2 | aagA aggR aap chuA irp2 set1 pic |
| 278-1 | AA/DA | aggR aap irp2 set1 pic |
See reference 14.
aggA and aafA encode the respective structural subunits for AAF/I and AAF/II structures; aap encodes antiaggregation protein dispersin; aggR encodes AAF and aap transcriptional activator; chuA and fyuA encode outer membrane receptors for heme transport and yersiniabactin siderophore, respectively; iucA encodes an aerobactin synthesis gene; shf enhances biofilm formation. For more information, see references 14, 22, and 47.
ND, not determined.
Visualization of bacteria inside colonized nematodes.
Green fluorescent protein (GFP)-expressing plasmid pGFP (Clontech) was electroporated into ampicillin-sensitive EAEC strains 042 and 17-2, as well as control strain OP50. For ampicillin-resistant strain 101-1, plasmid pHTK4, expressing dsRed from a trimethoprim-resistant vector, was employed (61). Bacterial fluorescent proteins were plated on LB plates containing 20 μg/μl of ampicillin or 200 μg/μl trimethoprim and were incubated at 37°C for 12 to 24 h preseeding. Worms fed for 3 days, at which point they were washed briefly in M9 buffer and placed on a slide containing a thin layer of 1% agarose. Fluorescent microscopy was used to visualize GFP- or dsRed-expressing bacteria within live worms as described by Tan et al. (57).
HEp-2 adherence assay.
The method originally described by Cravioto et al. (13) was used with modifications necessary for delineating aggregative adherence stipulated by Vial et al. (66). HEp-2 cell monolayers were cultured overnight in 8-well chamber slides (for qualitative tests) or 24-well plates (for quantitative assays) to 50% confluence in high-glucose Dulbecco modified Eagle medium (DMEM) with fetal bovine serum, streptomycin, and penicillin (Invitrogen). Cultures of test bacteria were incubated in LB at 37°C without shaking overnight. On the day of the adherence assay, the HEp-2 cells were washed three times with phosphate-buffered saline (PBS) and then refreshed with high-glucose DMEM containing 1% mannose (without fetal bovine serum and antibiotics). Then, 10 μl of overnight bacterial culture was added to each well. The infected cells were incubated for 3 h at 37°C in 5% CO2; then, the culture medium was aspirated and each well was washed three times with PBS. The cells were fixed for 20 min with 70% methanol and then stained for 20 min with a 1:40 dilution of Giemsa in PBS. Adherence patterns were observed using oil immersion light microscopy at a ×1,000 magnification. For quantitative adherence assays, as described by Torres et al. (62), after infection and incubation of monolayers, wells containing infected HEp-2 cells were washed three times with PBS. Each well was treated with 200 μl of 0.1% Triton X-100 in PBS for 15 min, and then serial dilutions of the detached and lysed sample were plated out for viable counting on LB, with antibiotics if appropriate. Two separate assays were performed in quadruplicate, and within-assay viable counts were compared statistically by an unpaired Student t test.
RESULTS
EAEC isolate 101-1 from an outbreak in Japan harbors a cadAB deletion.
We originally hypothesized that gene loss may contribute to heterogeneity in EAEC virulence and that since cadaverine, the product of lysine decarboxylase, interferes with Set1 toxigenicity in Shigella, the cad genes might be inactivated in EAEC strains that have set1 genes. We screened 49 EAEC strains for the cadABC genes by PCR and lysine decarboxylase activity. Thirteen of these strains have previously been reported to carry the set1A gene (14, 44), and the presence of this gene in these strains was confirmed in this study. Forty-six (94%) of the 49 EAEC strains tested, including all the set1-positive strains, possessed an intact and functional cadAB operon (see Table S2 in the supplemental material). Therefore, we conclude that, although many EAEC strains have the Shigella enterotoxin genes, set1-positive EAEC strains do not have the pathoadaptive deletion that has previously been reported to enhance the Set1 activity in Shigella (34).
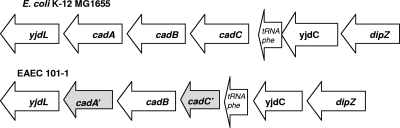
We identified only three EAEC strains, all set1 negative, from which the cadAB genes could not be amplified. These lysine decarboxylase-negative isolates were from different geographic locations and therefore were epidemiologically unrelated (see Table S2 in the supplemental material). One of the three cadAB-negative strains, EAEC strain 101-1, was isolated during a large outbreak in Japan, involving over 2,600 schoolchildren, leading us to hypothesize that the cad deletion might be pathoadaptive. The lysine decarboxylase defect could not be restored in strain 101-1 by cloned cadAB genes from E. coli K-12 strain MG1655, and the cadC gene could also not be amplified from strain 101-1. The sequence of the yjdL-yjdC intergenic region, which contains cadABC in E. coli K-12 strain MG1655, was retrieved for strain 101-1 from the in-process genome (GenBank accession number NZ_AAMK00000000). From pairwise alignments with E. coli K-12 strain MG1655 DNA, we determined that strain 101-1 has a 30-bp deletion in the cadA gene as well as a larger deletion in cadC, the gene product of which is responsible for activating cadAB (Fig. 1). The cadA clone used to complement pathoadaptive deletions in Shigella (pCADA) (34) was introduced into EAEC strain 101-1. In this clone, the lysine decarboxylase gene is under the control of the lac promoter. Upon induction with IPTG (isopropyl-β-d-thiogalactopyranoside), the trans-complemented derivative of strain 101-1 demonstrated lysine decarboxylase activity in vitro. The finding that a strain that was responsible for a significant outbreak tested cadAB negative prompted in vivo studies on this strain to determine whether the cadAB deletion was pathoadaptive, as it is in Shigella and E. coli O157.
FIG. 1.
Schematic of the yjdC-yjdL intergenic region in E. coli K-12 MG1655 and EAEC strain 101-1. This region of the chromosome contains the cadABC genes in MG1655. Strain 101-1 is identical to MG1655 at this locus with the exception of a 5′ 30-nucleotide deletion in cadA and a 558-nucleotide deletion in cadC. Genes harboring inactivating deletions are shaded.
EAEC strains colonize C. elegans and are virulent in the slow-kill assay.
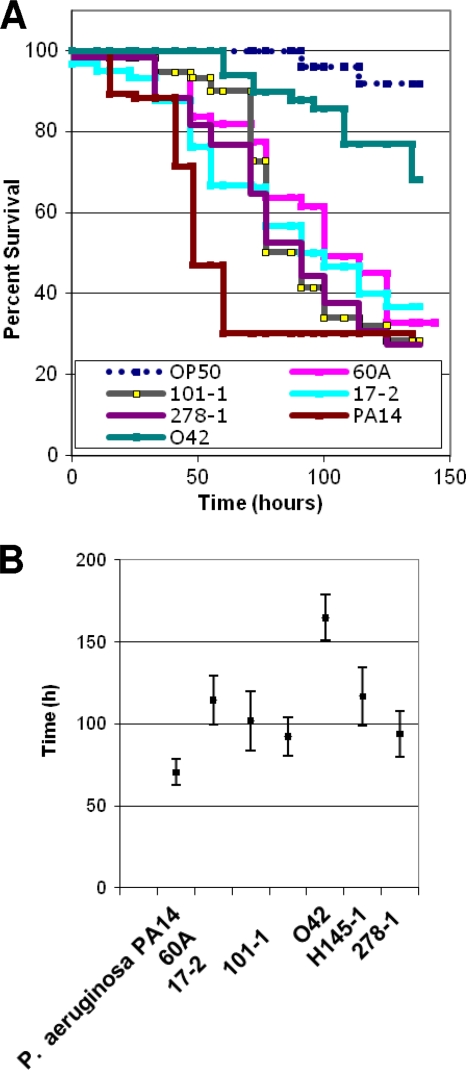
We fed six well-studied EAEC strains (Table 2) to C. elegans in a slow-kill assay essentially similar to that described by Tan et al. (57) and Aballay et al. (2). Consistent with documented heterogeneity of EAEC virulence factors, different EAEC strains showed various levels of virulence. As illustrated for five strains in Fig. 2A, EAEC strains were significantly more lethal for worms than control strain OP50. Outbreak strain 101-1 from Japan, strain 17-2 from Chile, and isolate 60A from Mexico were the most virulent in the slow-kill model. Prototypical EAEC strain 042 was less virulent to C. elegans than other EAEC strains tested in this study, but it did show demonstrable and significant activity compared to that of nonpathogenic E. coli strain OP50 (Fig. 2).
FIG. 2.
Survival of C. elegans fed with EAEC strains. (A) Kaplan-Meier plots for worms fed on OP50, P. aeruginosa PA14, and five EAEC strains. (B) Median time to death for C. elegans fed with P. aeruginosa or one of six EAEC strains. Each experiment used 40 to 60 worms for each strain, and survival data were compared statistically by the Kaplan-Meier method (7) using the PEPI version 4.0 SURVIVAL program. Survival of all EAEC strains was significantly less than that of OP50 (P < 0.05), which had a median time to death of >400 h.
To show that increased death rates are associated with EAEC colonization of the worm intestine, worms were fed with EAEC strains 17-2 and 042, which expressed GFP under the control of the lac promoter. Worms fed on these strains in the presence of IPTG were observed by fluorescence microscopy. As shown in Fig. S1 in the supplemental material, bacteria could be located along the entire length of the intestine when worms were fed on EAEC strains. However, OP50 bacteria bearing the same plasmid were found only in the anterior pharyngeal region and no colonization of the gut was observed, indicating that unlike EAEC, OP50 does not colonize distal to the grinder.
The cadAB deletion in EAEC strain 101-1 is pathoadaptive in the C. elegans slow-kill model.
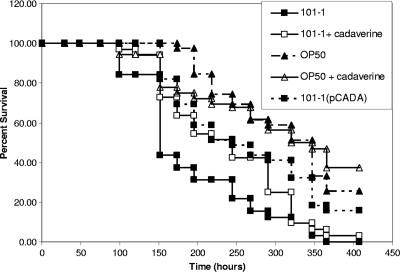
We compared slow killing of C. elegans by EAEC strain 101-1 alone and strain 101-1 complemented with pCADA to determine whether lysine decarboxylase capability was attenuating. As shown in Fig. 3, the lysine decarboxylase-complemented strain, induced with IPTG, was significantly attenuated compared to the wild-type strain (P = 0.001) but was still more virulent than nonpathogenic strain OP50 (P = 0.001). We additionally observed the effects on the slow-kill profile of strain 101-1 when 300 μM cadaverine was added to the culture medium in order to determine whether exogenous cadaverine would produce the attenuation seen when lysine decarboxylase activity was restored. Because cadaverine slowed bacterial growth slightly, we also tested strain OP50 in the presence and absence of cadaverine for control purposes. Cadaverine slightly but insignificantly increased the death rate of C. elegans fed OP50 (P > 0.05). In contrast, cadaverine slightly attenuated strain 101-1 virulence but these differences were again not significant (P > 0.05) (Fig. 3).
FIG. 3.
Survival of C. elegans fed with wild-type EAEC outbreak strain 101-1, 101-1 in the presence of exogenous cadaverine, and 101-1 complemented with pCADA. Strain OP50 was used as a negative control in the presence and absence of cadaverine. The pCADA-bearing derivative was significantly attenuated compared to the wild-type 101-1 strain (P = 0.001). However, the comparison of strain 101-1 fed in the presence and absence of cadaverine and OP50 fed in the presence and absence of cadaverine did not show a significant difference in survival.
Restoration of the lysine decarboxylase defect in EAEC strain 101-1 reduces epithelial cell adherence.
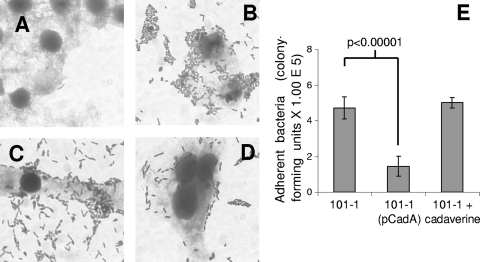
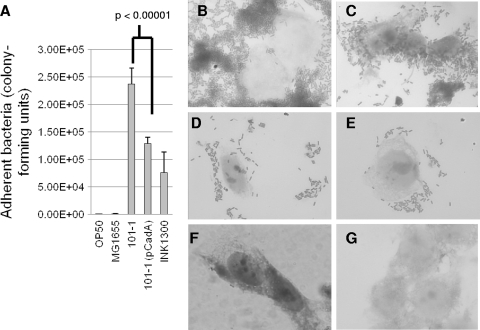
Recent reports have demonstrated that cad deletions enhance adherence of EHEC and other pathogenic E. coli strains (31, 63, 65). We therefore measured adherence of EAEC strain 101-1 and its IPTG-induced pCADA trans-complement as well as strain 101-1 in the presence of 300 μM cadaverine. As shown in Fig. 4, exogenous cadaverine had no significant effect on the adherence of strain 101-1 but complementation with pCADA decreased the number of bacteria adhering to HEp-2 cell monolayers (P < 0.00001).
FIG. 4.
Bacterial adherence to HEp-2 cell monolayers after 3-h infections. (A) E. coli OP50. (B) EAEC strain 101-1. (C) Strain 101-1 in the presence of 300 μM cadaverine. (D) Strain 101-1 complemented with plasmid pCADA and induced with IPTG. (E) Adherent bacteria quantified by viable counting. The differences between 101-1 and 101-1(pCADA) were significant at P < 0.00001.
Insertion of the cad genes into the chromosome attenuates strain 101-1.
Because of the possibility of gene-dosage effects, we created a derivative of EAEC strain 101-1 with the cadCBA genes from E. coli K-12 strain MG1655 inserted into its chromosome. The genes were inserted at attTn7, between pstS and glmS. The resulting derivative, INK1300, was lysine decarboxylase positive. As shown in Fig. 5, like the pCADA-complemented version of strain 101-1, INK1300 was deficient in adherence to cultured HEp-2 cells. INK1300 was also significantly attenuated in the C. elegans model (P = 0.001) (Fig. 6).
FIG. 5.
Adherence of 101-1 and its cad cis-complement to cultured HEp-2 cells. (A) Adherence quantified by viable counting. Adherence of INK1300 and adherence of 101-1(pCADA) are not significantly different from one another, but both adhere to a lesser degree than wild-type strain 101-1 (P < 0.00001). (B to G) Bacterial adherence to cultured HEp-2 cells after 3-h infections. (B) Prototypical EAEC strain 042. (C) Outbreak strain 101-1. (D) 101-1(pCADA). (E) INK1300. (F) OP50. (G) Uninfected HEp-2 cells.
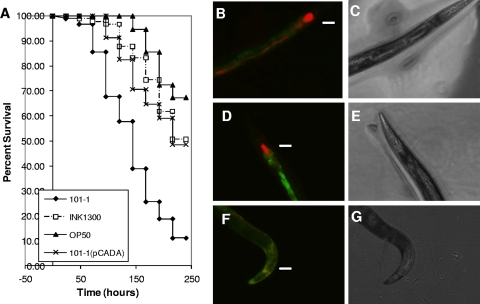
FIG. 6.
(A) C. elegans survival rates of wild-type EAEC outbreak strain 101-1, 101-1 complemented with pCADA, and INK1300, the derivative of 101-1 with the cad operon integrated into the chromosome. Strain OP50 was used as a negative control. Both strain INK1300 and 101-1 bearing pCADA were significantly attenuated compared to the wild-type 101-1 strain (P = 0.001). The observed differences between strains fed on INK1300 and 101-1(pCADA) did not show statistical significance. (B to G) Visualization of bacterial strains OP50, 101-1, and INK1300 expressing dsRed from pHKT4 inside infected C. elegans. (B) Fluorescence of bacteria within a worm that has used 101-1(pHTK4) as a food source. Note intense colonization of the pharynx and intact bacteria present inside the worm anterior to the grinder (marked with an arrow in panels B, D, and F). Autofluorescence of the lumen is visualized in green. (D) Fluorescence of a worm that has used INK1300(pHTK4) as a food source. As with 101-1, the pharynx is colonized but there are fewer bacteria posterior to the grinder. (F) Fluorescence of a worm that has used OP50 as a food source, showing very little pharyngeal colonization and no detectable red fluorescence distal to the grinder. (C, E, and G) Light field views of worms in panels B, D, and F, respectively. Photomicrographs were taken 3 days postseeding with a fluorescence microscope at ×100 magnification.
DISCUSSION
The complete repertoire of EAEC virulence factors is far from characterized, in part due to the absence of an accessible model system. The impracticable human volunteer challenge model (41) is the only system that reproduces EAEC disease and colonization. A number of researchers have attempted to develop alternative disease models for EAEC. For example, human intestinal explants and T84 cells have been used to model the adhesive and cytotoxic effects on intestinal mucosa, while an abiotic intestinal simulator (in vitro anaerobic continuous culture system) and a gnotobiotic piglet model have been used to study virulence gene expression and diarrheagenic effects (26, 43, 64). Mouse models have been less successful but have recently been used to study colonization (25). With the exception of tissue culture-based models, EAEC disease models are highly specialized and therefore performed in only one or two labs. Because the EAEC category is heterogeneous, a simple, practical, economical, and potentially high-throughput model that can be used for preliminary screens is essential for studying host-pathogen interactions. Conveniently, innate antimicrobial immune responses are conserved in plant, vertebrate, and invertebrate species, enabling researchers to make inferences about host-pathogen interactions from a simple and easily manipulated invertebrate (1, 40, 49).
The well-studied nematode C. elegans is able to mount a nonspecific innate immune response to pathogens (3, 49). The initial and best-characterized C. elegans host-pathogen interaction is with Pseudomonas aeruginosa. Worms fed on bacteria that have been grown on standard nematode growth media (NGM) die over a period of 2 to 3 days by a method that is referred to as “slow killing” and is a consequence of colonization of the worms’ intestinal lumen (33, 57). Since Tan et al. (57) developed this paradigm in 1999, many other bacterial pathogens have been identified as slow killers of C. elegans, including various Streptococcus, Burkholderia, Salmonella, and Yersinia species, as well as enteropathogenic E. coli (2, 21, 36, 40, 57, 59, 60). Virulence is usually mediated by toxin secretion, biofilm formation, or colonization. We have shown that EAEC strains kill C. elegans when used as a food source, most likely by means of an infection-like process, and that EAEC strains colonize the distal C. elegans intestine, whereas nonpathogenic E. coli strains do not. This work provides the groundwork for the future identification and characterization of putative EAEC virulence factors. Furthermore, by demonstrating pathoadaptiveness of a cad deletion, a paradigm that has been documented for other intestinal pathogens using other systems, this study demonstrates that the C. elegans model is well suited for testing colonization and pathogenesis-related hypotheses.
Earlier studies of EAEC heterogeneity have focused on loci that are absent in nonpathogenic E. coli, but loss, as well as gain, of genetic material can be associated with evolution to virulence. Maurelli et al. demonstrated this by showing that deletion of the cad genes, encoding lysine decarboxylase, enhances the virulence of Shigella and enteroinvasive E. coli (16, 34). Shigellae have also lost function of the nadA and nadB genes, which are required for the synthesis of NAD. Quinolinate, which is produced sequentially from l-aspartate by the product of nadA, nadB, and nadC, is also an inhibitor of Shigella virulence (50, 51). Similarly, Sun et al. showed that natural deletion of rcsA in Yersinia pestis promotes biofilm formation and accounts for the flea-blocking phenotype that is essential for efficient transmission of this deadly vector-borne pathogen (56). The rcsA-negative repressor of biofilm formation is intact and active in E. coli and in Yersinia pseudotuberculosis, which is believed to be ancestral to Y. pestis. Most recently, expression of the lac repressor, which has been lost by Salmonella enterica but not nonpathogenic Salmonella bongori or E. coli, has been shown to lead to repression of Salmonella pathogenicity island-2 genes required for intramacrophage survival (18). Thus, bacterial pathogens commonly harbor pathoadaptive deletions, and their absence in earlier microbiology literature most likely relates to the challenges associated with their discovery.
The cad deletion, which prevents the bacteria from converting lysine into cadaverine and thereby reduces acid resistance, is the best-studied pathoadaptive deletion. There are a number of mechanisms by which this deletion has allowed bacteria to more efficiently infect its host. In Shigella, the deletion specifically promotes Shigella enterotoxin activity because cadaverine inhibits the activity of this toxin (34). Additionally, the deletion promotes intercellular dissemination since cadaverine has been shown to reduce the capacity of Shigella to escape from the phagolysosome (20). cad deletions have also been shown to enhance adherence by EHEC and other diarrheagenic E. coli strains via an unknown mechanism (31, 63, 65).
Observing that EAEC strains commonly harbor the setA1 gene, we originally hypothesized that set1-positive strains might harbor cad deletions. However, we found that all EAEC strains tested in this study that possess the set1A gene also have intact and functional cad genes. Thus, our starting hypothesis was not supported. Given that negative selection due to Set1 does not appear to occur in EAEC, it is likely that Shigella enterotoxins play a less significant role in the virulence of EAEC than in Shigella. Our data instead support another hypothesis: that for some strains, such as the outbreak strain 101-1, enhanced host colonization is the selective advantage conferred by the EAEC pathoadaptive deletion. Strain 101-1 encodes a putative unique bundle-forming pilus, but the in-process genome sequence of this strain is yet to uncover any other loci that could account for the hypervirulence of this strain (52). Using the worm model, we showed that EAEC strain 101-1 colonizes the gut of C. elegans, leading to a slow-death phenotype. The lethality of strain 101-1 is attenuated in the C. elegans model when a functional cadA gene is expressed in trans. Additionally, adherence of strain 101-1 to HEp-2 cells was reduced when the cadA gene was expressed in the strain. As has been reported for EHEC strains with cad deletions, addition of extraneous cadaverine did not affect adherence or virulence (63, 65). This suggests that cadaverine produced by other bacteria may be insufficient to ameliorate the consequences of an intestinal infection caused by strain 101-1. The exact mechanism by which the deletion of the cad operon produces enhanced adherence remains unknown and is an important area for future research.
Prior to this study, it had not been confirmed that the lysine decarboxylase deletion, or any other pathoadaptive mutation, could be complemented in single copy under the control of the native promoter from a nonpathogen. All previous complementation experiments have restored the deleted genes in multicopy, often under the control of inducible promoters. Therefore, attenuation could, wholly or in part, be attributed to gene dosage effects. In this study, we constructed a cis-complement strain bearing a single copy of cadA, and its activator cadC, on the chromosome. This strain, like the trans-complement of EAEC strain 101-1, was attenuated in the C. elegans model, demonstrating for the first time that attenuation following restoration of pathoadaptive mutations is not due to overproduction of lysine decarboxylase or other dosage effect. The cis-complement strain INK1300 adhered in a pattern similar to and to an extent similar to that of 101-1 complemented with pCADA, confirming that complementation of the cadA and cadC deletions in single and multicopy produces similar effects (Fig. 5).
Similarly to our discovery of a cad deletion in an outbreak EAEC strain, a recent report describes an outbreak due to an EHEC strain bearing a pathoadaptive mutation (10). This and the case of EAEC strain 101-1 suggest that deletion of cad genes may be an evolutionary route to hypervirulence that could precipitate outbreaks. The genomes of three EAEC strains evaluated in this study, including strain 101-1, which harbors the pathoadaptive mutation, are completed (strain 042) (11) or in progress (strains 17-2 and 101-1). The development of a C. elegans model provides a simple and adaptable system for testing colonization and pathogenesis-related hypotheses with these and other EAEC strains.
Supplementary Material
Acknowledgments
Development of a C. elegans colonization model for EAEC was supported through NSF awards RUI 0516591 (to I.N.O.) and RUI 0614740 (to P.M.M.). We thank the Howard Hughes Medical Institute for support of undergraduate multicultural science program researcher J.H. at Haverford College. I.N.O. is a Branco Weiss fellow of the Society-in-science, ETHZ, Zürich, Switzerland.
We are grateful to Thomas Whittam for supplying strain 101-1 and Fred Ausubel for strain PA14 and helpful suggestions. Anthony Maurelli and Howard Ceri kindly supplied the cadA-expressing clone, pCADA, and dsRed-expressing plasmid, pHTK, respectively. pGRG36 was a gift from Nancy Craig. We thank Robert Loudon, Jay Mellies, and David Rasko for helpful discussions and Julia Durante for technical assistance. We are grateful to the Bio300 classes of Spring 2003 and Spring 2005, who, respectively, performed preliminary experiments to test the “black hole” hypothesis in EAEC genomes and tested the reproducibility of the C. elegans colonization model.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 28 June 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aballay, A., and F. M. Ausubel. 2002. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr. Opin. Microbiol. 5:97-101. [DOI] [PubMed] [Google Scholar]
- 2.Aballay, A., P. Yorgey, and F. M. Ausubel. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10:1539-1542. [DOI] [PubMed] [Google Scholar]
- 3.Alegado, R. A., M. C. Campbell, W. C. Chen, S. S. Slutz, and M. W. Tan. 2003. Characterization of mediators of microbial virulence and innate immunity using the Caenorhabditis elegans host-pathogen model. Cell. Microbiol. 5:435-444. [DOI] [PubMed] [Google Scholar]
- 4.Bernier, C., P. Gounon, and C. Le Bouguenec. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 70:4302-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhan, M. K., P. Raj, M. M. Levine, J. B. Kaper, N. Bhandari, R. Srivastava, R. Kumar, and S. Sazawal. 1989. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J. Infect. Dis. 159:1061-1064. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava, S., B. B. Johnson, J. Hwang, T. A. Harris, A. S. George, A. Muir, J. Dorff, and I. N. Okeke. 2009. The heat-resistant agglutinin 1 is an accessory enteroaggregative Escherichia coli colonization factor. J. Bacteriol. 191:4934-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland, J. M., and D. G. Altman. 1998. Survival probabilities (the Kaplan-Meier method). Br. Med. J. 317:1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisen, N., C. Struve, F. Scheutz, K. A. Krogfelt, and J. P. Nataro. 2008. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect. Immun. 76:3281-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderon, V. E., Q. Chang, M. McDermott, M. B. Lytle, G. McKee, K. Rodriguez, D. A. Rasko, V. Sperandio, and A. G. Torres. 2009. Outbreak caused by cad-negative Shiga toxin-producing Escherichia coli O111, Oklahoma. Foodborne Pathog. Dis. 7:107-109. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri, R. R., M. Sebaihia, J. L. Hobman, M. A. Webber, D. L. Leyton, M. D. Goldberg, A. F. Cunningham, A. Scott-Tucker, P. R. Ferguson, C. M. Thomas, G. Frankel, C. M. Tang, E. G. Dudley, I. S. Roberts, D. A. Rasko, M. J. Pallen, J. Parkhill, J. P. Nataro, N. R. Thomson, and I. R. Henderson. 2010. Complete genome sequence and comparative metabolic profiling of the prototypical enteroaggregative Escherichia coli strain 042. PLoS One 5:e8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobeljic, M., B. Miljkovic-Selimovic, D. Paunovic-Todosijevic, Z. Velickovic, Z. Lepšanovic, N. Zec, D. Savic, R. Ilic, S. Konstantinovic, B. Jovanovic, and V. Kostic. 1996. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol. Infect. 117:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cravioto, A., R. Gross, S. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional enteropathogenic serotypes. Curr. Microbiol. 3:95-99. [Google Scholar]
- 14.Czeczulin, J., T. Whittam, I. Henderson, F. Navarro-Garcia, and J. Nataro. 1999. Phylogenetic analysis of virulence genes in enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun. 67:2692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czeczulin, J. R., S. Balepur, S. Hicks, A. Phillips, R. Hall, M. H. Kothary, F. Navarro-Garcia, and J. P. Nataro. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 65:4135-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day, W. A., Jr., R. E. Fernandez, and A. T. Maurelli. 2001. Pathoadaptive mutations that enhance virulence: genetic organization of the cadA regions of Shigella spp. Infect. Immun. 69:7471-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley, E. G., C. Abe, J. M. Ghigo, P. Latour-Lambert, J. C. Hormazabal, and J. P. Nataro. 2006. An IncI1 plasmid contributes to the adherence of the atypical enteroaggregative Escherichia coli strain C1096 to cultured cells and abiotic surfaces. Infect. Immun. 74:2102-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eswarappa, S. M., G. Karnam, A. G. Nagarajan, S. Chakraborty, and D. Chakravortty. 2009. lac repressor is an antivirulence factor of Salmonella enterica: its role in the evolution of virulence in Salmonella. PLoS One 4:e5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falkow, S. 1958. Activity of lysine decarboxylase as an aid in the identification of Salmonellae and Shigellae. Tech. Bull. Regist. Med. Technol. 28:106-108. [PubMed] [Google Scholar]
- 20.Fernandez, I. M., M. Silva, R. Schuch, W. A. Walker, A. M. Siber, A. T. Maurelli, and B. A. McCormick. 2001. Cadaverine prevents the escape of Shigella flexneri from the phagolysosome: a connection between bacterial dissemination and neutrophil transepithelial signaling. J. Infect. Dis. 184:743-753. [DOI] [PubMed] [Google Scholar]
- 21.Fuhrman, L. E., K. V. Shianna, and A. Aballay. 2008. High-throughput isolation and mapping of C. elegans mutants susceptible to pathogen infection. PLoS One 3:e2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiyama, R., J. Nishi, N. Imuta, K. Tokuda, K. Manago, and Y. Kawano. 2008. The shf gene of a Shigella flexneri homologue on the virulent plasmid pAA2 of enteroaggregative Escherichia coli 042 is required for firm biofilm formation. Curr. Microbiol. 56:474-480. [DOI] [PubMed] [Google Scholar]
- 23.Harada, T., M. Hiroi, F. Kawamori, A. Furusawa, K. Ohata, K. Sugiyama, and T. Masuda. 2007. A food poisoning diarrhea outbreak caused by enteroaggregative Escherichia coli serogroup O126:H27 in Shizuoka, Japan. Jpn. J. Infect. Dis. 60:154-155. [PubMed] [Google Scholar]
- 24.Harrington, S. M., E. G. Dudley, and J. P. Nataro. 2006. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol. Lett. 254:12-18. [DOI] [PubMed] [Google Scholar]
- 25.Harrington, S. M., J. Sheikh, I. R. Henderson, F. Ruiz-Perez, P. S. Cohen, and J. P. Nataro. 2009. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 77:2465-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks, S., D. C. Candy, and A. D. Phillips. 1996. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect. Immun. 64:4751-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, D. B., J. P. Nataro, H. L. DuPont, P. P. Kamat, A. D. Mhatre, P. C. Okhuysen, and T. Chiang. 2006. Enteroaggregative Escherichia coli is a cause of acute diarrheal illness: a meta-analysis. Clin. Infect. Dis. 43:556-563. [DOI] [PubMed] [Google Scholar]
- 28.Huang, D. B., P. C. Okhuysen, Z. D. Jiang, and H. L. DuPont. 2004. Enteroaggregative Escherichia coli: an emerging enteric pathogen. Am. J. Gastroenterol. 99:383-389. [DOI] [PubMed] [Google Scholar]
- 29.Itoh, Y., I. Nagano, M. Kunishima, and T. Ezaki. 1997. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J. Clin. Microbiol. 35:2546-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joo, L. M., L. R. Macfarlane-Smith, and I. N. Okeke. 2007. Error-prone DNA repair system in enteroaggregative Escherichia coli identified by subtractive hybridization. J. Bacteriol. 189:3793-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jores, J., A. G. Torres, S. Wagner, C. B. Tutt, J. B. Kaper, and L. H. Wieler. 2006. Identification and characterization of “pathoadaptive mutations” of the cadBA operon in several intestinal Escherichia coli. Int. J. Med. Microbiol. 296:547-552. [DOI] [PubMed] [Google Scholar]
- 32.Lewis, J., and J. Fleming. 1995. Basic culture methods, p. 4-30. In H. Epstein and D. Shakes (ed.), Caenorhabditis elegans: modern biological analysis of an organism, vol. 48. Academic Press, San Diego, CA. [Google Scholar]
- 33.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 34.Maurelli, A. T., R. E. Fernandez, C. A. Bloch, C. K. Rode, and A. Fasano. 1998. “Black holes” and bacterial pathogenicity: a large genomic deletion that enhances the virulence of Shigella spp. and enteroinvasive Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 95:3943-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie, G. J., and N. L. Craig. 2006. Fast, easy and efficient: site-specific insertion of transgenes into enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellies, J. L., A. M. Barron, K. R. Haack, A. S. Korson, and D. A. Oldridge. 2006. The global regulator Ler is necessary for enteropathogenic Escherichia coli colonization of Caenorhabditis elegans. Infect. Immun. 74:64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng, S. Y., and G. N. Bennett. 1992. Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J. Bacteriol. 174:2659-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng, S. Y., and G. N. Bennett. 1992. Regulation of the Escherichia coli cad operon: location of a site required for acid induction. J. Bacteriol. 174:2670-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monteiro-Neto, V., S. Y. Bando, C. A. Moreira-Filho, and J. A. Giron. 2003. Characterization of an outer membrane protein associated with haemagglutination and adhesive properties of enteroaggregative Escherichia coli O111:H12. Cell. Microbiol. 5:533-547. [DOI] [PubMed] [Google Scholar]
- 40.Mylonakis, E., and A. Aballay. 2005. Worms and flies as genetically tractable animal models to study host-pathogen interactions. Infect. Immun. 73:3833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nataro, J. P., Y. Deng, S. Cookson, A. Cravioto, S. J. Savarino, L. D. Guers, M. M. Levine, and C. O. Tacket. 1995. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J. Infect. Dis. 171:465-468. [DOI] [PubMed] [Google Scholar]
- 42.Nataro, J. P., Y. Deng, D. R. Maneval, A. L. German, W. C. Martin, and M. M. Levine. 1992. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 60:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nataro, J. P., S. Hicks, A. D. Phillips, P. A. Vial, and C. L. Sears. 1996. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect. Immun. 64:4761-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okeke, I. N., A. Lamikanra, J. Czeczulin, F. Dubovsky, J. B. Kaper, and J. P. Nataro. 2000. Heterogeneous virulence of enteroaggregative Escherichia coli strains isolated from children in Southwest Nigeria. J. Infect. Dis. 181:252-260. [DOI] [PubMed] [Google Scholar]
- 45.Okeke, I. N., A. Lamikanra, H. Steinruck, and J. B. Kaper. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 38:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okeke, I. N., and J. P. Nataro. 2001. Enteroaggregative Escherichia coli. Lancet Infect. Dis. 1:304-313. [DOI] [PubMed] [Google Scholar]
- 47.Okeke, I. N., I. C. Scaletsky, E. H. Soars, L. R. Macfarlane, and A. G. Torres. 2004. Molecular epidemiology of the iron utilization genes of enteroaggregative Escherichia coli. J. Clin. Microbiol. 42:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pai, M., G. Kang, B. S. Ramakrishna, A. Venkataraman, and J. Muliyil. 1997. An epidemic of diarrhoea in south India caused by enteroaggregative Escherichia coli. Indian J. Med. Res. 106:7-12. [PubMed] [Google Scholar]
- 49.Pradel, E., and J. J. Ewbank. 2004. Genetic models in pathogenesis. Annu. Rev. Genet. 38:347-363. [DOI] [PubMed] [Google Scholar]
- 50.Prunier, A. L., R. Schuch, R. E. Fernandez, and A. T. Maurelli. 2007. Genetic structure of the nadA and nadB antivirulence loci in Shigella spp. J. Bacteriol. 189:6482-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prunier, A. L., R. Schuch, R. E. Fernandez, K. L. Mumy, H. Kohler, B. A. McCormick, and A. T. Maurelli. 2007. nadA and nadB of Shigella flexneri 5a are antivirulence loci responsible for the synthesis of quinolinate, a small molecule inhibitor of Shigella pathogenicity. Microbiology 153:2363-2372. [DOI] [PubMed] [Google Scholar]
- 52.Rasko, D. A., M. J. Rosovitz, G. S. Myers, E. F. Mongodin, W. F. Fricke, P. Gajer, J. Crabtree, M. Sebaihia, N. R. Thomson, R. Chaudhuri, I. R. Henderson, V. Sperandio, and J. Ravel. 2008. The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. J. Bacteriol. 190:6881-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 54.Sheikh, J., J. R. Czeczulin, S. Harrington, S. Hicks, I. R. Henderson, C. Le Bouguenec, P. Gounon, A. Phillips, and J. P. Nataro. 2002. A novel dispersin protein in enteroaggregative Escherichia coli. J. Clin. Invest. 110:1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith, H. R., T. Cheasty, and B. Rowe. 1997. Enteroaggregative Escherichia coli and outbreaks of gastroenteritis in UK. Lancet 350:814-815. [DOI] [PubMed] [Google Scholar]
- 56.Sun, Y. C., B. J. Hinnebusch, and C. Darby. 2008. Experimental evidence for negative selection in the evolution of a Yersinia pestis pseudogene. Proc. Natl. Acad. Sci. U. S. A. 105:8097-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor, L. A., and R. E. Rose. 1988. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 16:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tenor, J. L., and A. Aballay. 2008. A conserved Toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep. 9:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tenor, J. L., B. A. McCormick, F. M. Ausubel, and A. Aballay. 2004. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr. Biol. 14:1018-1024. [DOI] [PubMed] [Google Scholar]
- 61.Tomlin, K. L., S. R. Clark, and H. Ceri. 2004. Green and red fluorescent protein vectors for use in biofilm studies of the intrinsically resistant Burkholderia cepacia complex. J. Microbiol. Methods 57:95-106. [DOI] [PubMed] [Google Scholar]
- 62.Torres, A. G., J. A. Giron, N. T. Perna, V. Burland, F. R. Blattner, F. Avelino-Flores, and J. B. Kaper. 2002. Identification and characterization of lpfABCC′DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 70:5416-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres, A. G., R. C. Vazquez-Juarez, C. B. Tutt, and J. G. Garcia-Gallegos. 2005. Pathoadaptive mutation that mediates adherence of Shiga toxin-producing Escherichia coli O111. Infect. Immun. 73:4766-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzipori, S., J. Montanaro, R. M. Robins-Browne, P. Vial, R. Gibson, and M. M. Levine. 1992. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect. Immun. 60:5302-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vazquez-Juarez, R. C., J. A. Kuriakose, D. A. Rasko, J. M. Ritchie, M. M. Kendall, T. M. Slater, M. Sinha, B. A. Luxon, V. L. Popov, M. K. Waldor, V. Sperandio, and A. G. Torres. 2008. CadA negatively regulates Escherichia coli O157:H7 adherence and intestinal colonization. Infect. Immun. 76:5072-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vial, P. A., J. J. Mathewson, H. L. DuPont, L. Guers, and M. M. Levine. 1990. Comparison of two assay methods for patterns of adherence to HEp-2 cells of Escherichia coli from patients with diarrhea. J. Clin. Microbiol. 28:882-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.