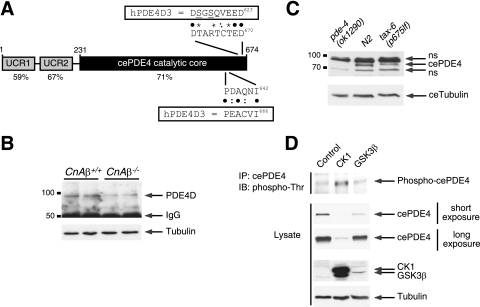
FIG. 8.
Evolutionarily conserved regulation of PDE4D degradation via calcineurin, CK1, and GSK3β. (A) Schematic illustration of C. elegans PDE4 (cePDE4). The locations of the potential phosphodegron and calcineurin docking motif are also shown. Sequence alignment and percent identity with human PDE4D (hPDE4D) are indicated. Bullets indicated conserved amino acid residues. Colons indicated similar amino acid residues. Asterisks indicated potential phosphorylation sites. (B) Extracts prepared from CnAβ−/− and CnAβ+/+ mouse skeletal muscle were subjected to immunoprecipitation and immunoblot analysis to determine the endogenous expression of PDE4D. The expression level of tubulin was used as a control. (C) Extracts prepared from N2 wild-type control C. elegans and loss-of-function calcineurin mutant worms [tax-6(p675lf)] were subjected to immunoblot analysis to determine the endogenous expression of cePDE4. Extracts prepared from PDE4 null worms [pde-4(ok1290)] were used to show the specificity of the cePDE4 antibody. The expression level of C. elegans tubulin was used as a control. (D) cePDE4 was transiently cotransfected with either CK1 or GSK3β into COS7 cells. Phosphorylation of cePDE4 was determined by immunoprecipitation (IP) using M2 monoclonal antibody against FLAG-tagged cePDE4 and subsequent immunoblotting (IB) analysis using phospho-Thr polyclonal antibodies. The expression levels of cePDE4, CK1, GSK3β, and tubulin are also shown.