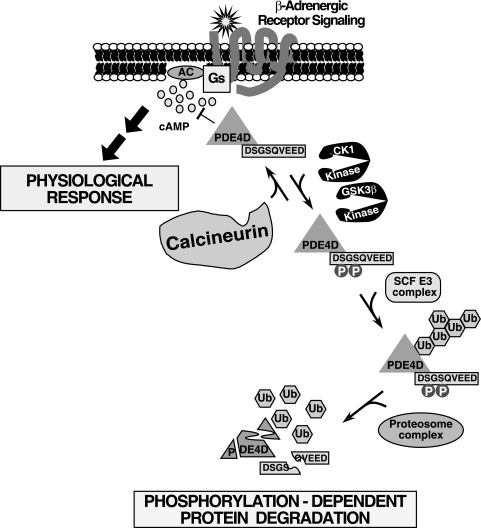
FIG. 9.
Model of the phosphatase calcineurin in the β-adrenergic signaling pathway via regulation of PDE4D degradation. PDE4D encompasses a DSGSQVEED phosphodegron. Phosphorylation at the phosphodegron by the protein kinases GSK3β and CK1 promotes PDE4D degradation, which is mediated by the SCF E3 ubiquitin (Ub) ligase complex. Ubiquitinated PDE4D was degraded by the proteosome complex. Conversely, dephosphorylation mediated by the phosphatase calcineurin opposes PDE4D degradation. Thus, phosphorylation-dependent degradation controls the expression level of PDE4D, which in turn regulates the intracellular concentration of the second messenger cAMP upon activation of the β-adrenergic receptor signaling pathway—a key component of the control of diverse physiological responses. The calcineurin/ubiquitin/PDE4D signaling axis is also evolutionarily conserved in C. elegans, indicating its important role in critical cellular processes to regulate cAMP duration. We propose that potentiation of the Gs-coupled signaling pathway due to sustained elevation of cAMP, which is a consequence of reduced PDE4D expression in the absence of calcineurin function, accounts for the metabolic complications (e.g., hyperlipidemia and hemodynamic dysregulations) found in transplant patients treated with CsA. AC, adenylyl cyclase.