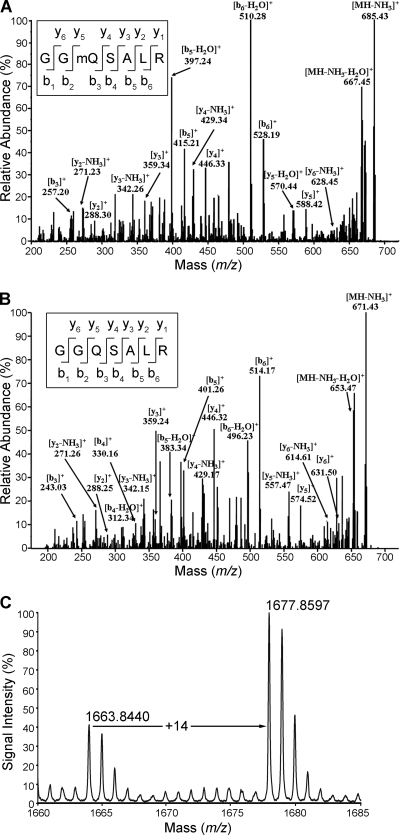
FIG. 1.
Identification of Gln185 methylation of eRF1 in vivo in mammalian cells. (A) ESI-MS/MS spectrum of methylated 702.34-Da fragments [heptapeptide GG(mQ)SALR, amino acids 183 to 189] from endogenous eRF1. eRF1 was purified by immunoprecipitation from the HEK-293T cell extract using anti-eRF1 polyclonal antibodies, separated by PAGE, and subjected to in-gel digestion with trypsin prior to ESI-MS/MS. The fragmentation pattern established the heptapeptide sequence indicated. (B) ESI-MS/MS spectrum of methylation-free 688.32-Da fragments from recombinant eRF1 purified from E. coli. (C) MALDI-TOF MS spectrum of endogenous eRF1 of HEK-293T cells. eRF1 was affinity purified with specific antibodies. The peak at m/z 1,663.84 is from the unmethylated peptide (sequence TVDLPKKHGRGGQSAL, amino acids 173 to 188) generated from chymotrypsin digestion. The peak at m/z 1,677.86 represents the methylated peptide.