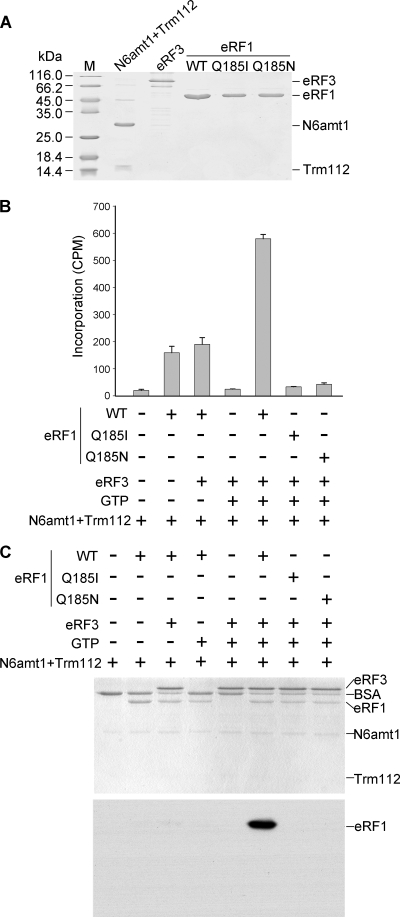
FIG. 2.
Characterization of the in vitro methylation activity of recombinant N6amt1. (A) Recombinant proteins used for the methylation assay. 6×His-tagged N6amt1 coexpressed FLAG-Trm112, GST-eRF3, and wild-type (WT) and mutant 6×His-eRF1 proteins were purified from E. coli and examined by Coomassie staining. (B) Methyltransferase activity detected by scintillation counting. eRF1 substrates were incubated with N6amt1 in the presence or absence of other factors using [methyl-3H]AdoMet as the methyl donor, and the proteins were then precipitated by trichloroacetic acid (TCA) for the measurement of methyl-3H incorporation. Averages from three independent experiments ± standard deviations are shown. (C) Methyl-transferase activity detected by fluorography. After the methylation reaction in the presence of radioactive [methyl-3H]AdoMet, the proteins were separated by SDS-PAGE and visualized by Coomassie staining (upper panel), and the same gel was subjected to fluorography (lower panel). Note that only the wild-type eRF1 was radioactively labeled.