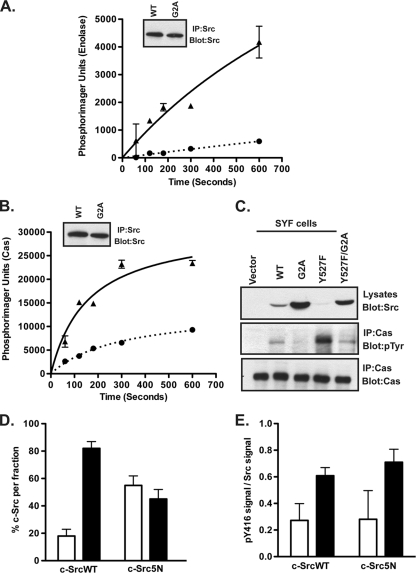
FIG. 6.
Nonmyristoylated c-Src exhibits reduced kinase activity. (A and B) COS-1 cells were transfected with WT or G2A c-Src constructs. At 24 h posttransfection, c-Src was immunoprecipitated and subjected to an in vitro kinase assay using enolase or purified p130Cas. The amounts of phosphorylated enolase (A) and Cas (B) were quantified by phosphorimaging and plotted as a function of time. Triangles = WT; circles = G2A mutant. Average data for two independent experiments are shown. Error bars indicate standard deviations. Insets show that equal amounts of c-SrcWT and c-SrcG2A were immunoprecipitated. (C) SYF cells transfected with WT or mutant c-Src constructs were lysed at 24 h posttransfection, and endogenous Cas was immunoprecipitated. Lysates and immunoprecipitated samples were analyzed by Western blotting with the indicated antibodies. (D and E) 3T3 cells stably expressing c-SrcWT and c-Src5N were subjected to cell fractionation. Equal volumes of the S100 and P100 fractions were analyzed by Western blotting, using anti-Src (GD11) (D) or anti-phospho-c-Src (Y416) (E) antibody, and were quantified using densitometry. Combined data for two independent experiments are shown. Open bars = S100; closed bars = P100.