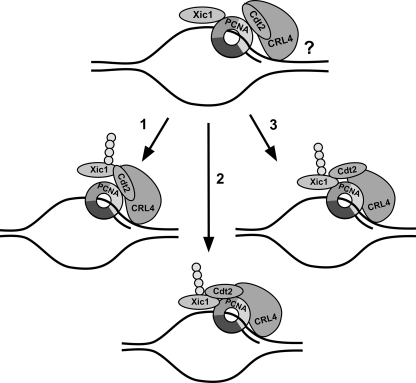
FIG. 10.
Model for PCNA-mediated Xic1 ubiquitination by CRL4Cdt2. In our working model, we hypothesize that Xic1 and Cdt2 can associate to different subunits of the PCNA trimer in the absence of DNA. We postulate that in the absence of DNA, the binding of PCNA and Cdt2 to Xic1 and the binding of Xic1 and Cdt2 to monomeric PCNA may be mutually exclusive. It is unclear whether the CRL4 ubiquitin ligase can associate with DNA independently or what role this might play in CRL4 substrate ubiquitination. We propose the following three possible scenarios for Xic1 ubiquitination following the chromatin recruitment of PCNA in association with Xic1 and Cdt2 (top) and a proposed PCNA conformational shift: (1) Xic1 dissociates from PCNA and binds to the N-terminal domain of PCNA-bound Cdt2, resulting in Xic1 ubiquitination; (2) chromatin-bound PCNA mediates a stable interaction among Xic1, Cdt2, and PCNA on DNA, resulting in Xic1 ubiquitination; and (3) Cdt2 dissociates from PCNA and binds to Xic1, resulting in Xic1 ubiquitination.