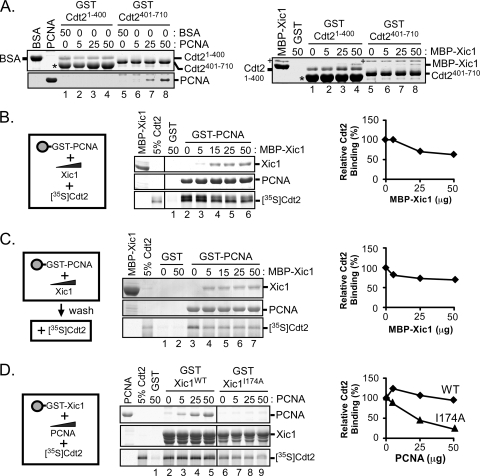
FIG. 9.
PCNA directly interacts with the C-terminal domain of XCdt2. (A) GST pulldown assay. Bacterially expressed GST, GST-XCdt21-400, or GST-XCdt2401-710 was bound to glutathione-Sepharose and incubated with purified XPCNA (0, 5, 25, and 50 μg) or bovine serum albumin (BSA; 0 and 50 μg) (left) as indicated and MBP-Xic1 (0, 5, 25, and 50 μg) (right), followed by staining with Coomassie blue. Protein bands were identified by mass spectrometry and are labeled accordingly. Several bacterial contaminants were identified. “+” was identified as the bacterial DnaK protein, and “*” was identified as the bacterial GroEL protein. (B) GST pulldown and competitive binding assay. Bacterially expressed GST or GST-PCNA (5 μg) was bound to glutathione-Sepharose beads and incubated with 0 to 50 μg of purified MBP-Xic1 or GST as indicated and 35S-labeled wild-type XCdt2. (C) GST pulldown and competition study. GST or GST-PCNA was bound to glutathione-Sepharose beads and incubated with 0 to 50 μg of purified MBP-Xic1 as indicated. Following a washing step, samples were incubated with 35S-labeled XCdt2. (D) GST pulldown assay and competitive binding assay. GST, GST-Xic1WT, or GST-Xic1I174A bound to glutathione-Sepharose beads was incubated with 0 to 50 μg of purified XPCNA and 35S-labeled wild-type XCdt2. (B to D) Samples were analyzed by Coomassie blue staining and phosphorimaging. (Left) Schematic representation of proteins analyzed in binding assays. (Right) The average relative Cdt2 binding values [relative Cdt2 binding (%)] of results from at least 2 independent experiments are shown, where the “zero competitor” value was normalized to 100%.