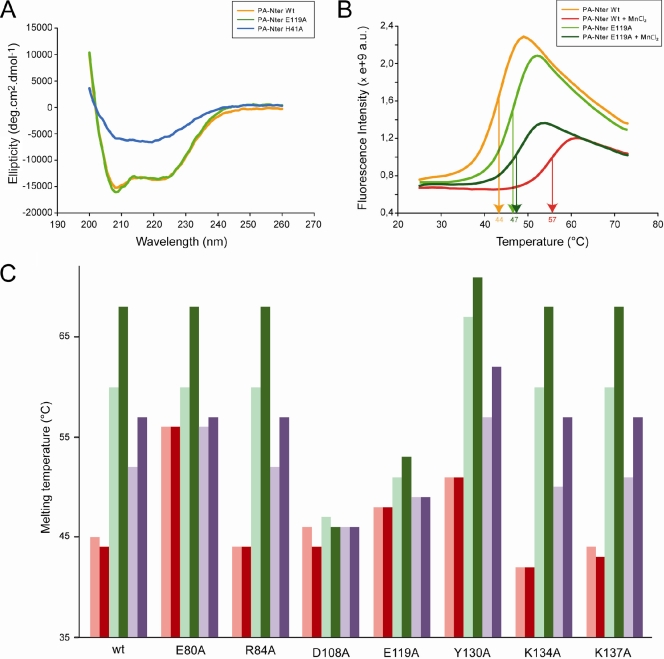
FIG. 2.
Biophysical characterization of PA-Nter mutants. (A) Circular dichroism on wt PA-Nter and two of the eight mutants. CD spectra of the wt, H41A, and E119A PA-Nters are in orange, blue, and green, respectively. (B) Thermal stabilization by MnCl2. Thermal shift assays on wt and E119A PA-Nters were done in the presence (red and dark green, respectively) or absence (orange and green, respectively) of 0.5 mM MnCl2. (C) Effects of metals and DPBA binding on the thermal stability. Thermal shift assays to test the metal ion stabilization were performed on wt PA-Nter and the corresponding mutants in the presence (dark colors) and absence (light colors) of DPBA. The proteins were incubated with 0.1 mM MnCl2 (green) or 5 mM MgCl2 (violet) or without any metal (red). All experiments were repeated at least three times on different occasions and with different protein preparations. The results were identical when the same experimental conditions were used.