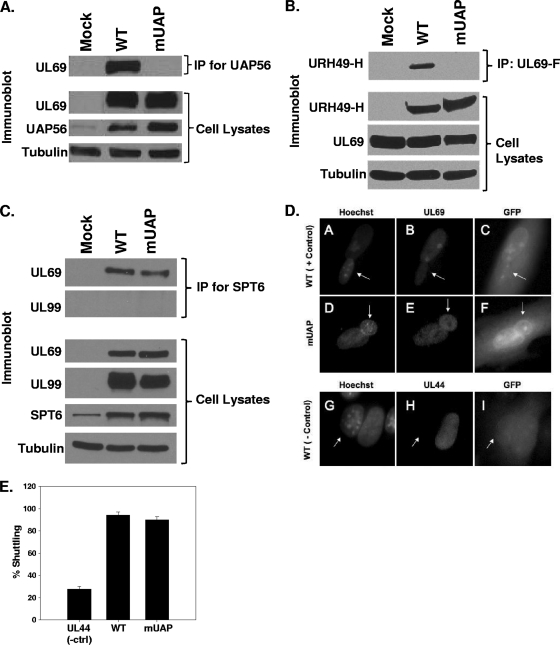
FIG. 2.
Interaction of WT and mutant UL69 with UAP56 and Spt6. (A through C) HFF cells were mock infected or infected with WT virus or ADmUAPUL69 (mUAP) virus at an MOI of 3 PFU/cell (A) or cotransfected with expression constructs expressing HA-tagged URH49 (URH49-H) and either FLAG-tagged WT UL69 or mUAPUL69 (mUAP) (B). Lysates were prepared 72 h postinfection or transfection and incubated with antibody against UAP56 (A), the FLAG epitope (B), or Spt6 (C). Immune complexes were collected via protein-A/G-agarose beads, separated by SDS-PAGE, transferred to membranes, and probed for UL69 or URH49 via Western blotting. Cell lysates were also examined for UL69, UAP56, URH49, and Spt6 expression by Western blot analysis. α-Tubulin was included as an internal loading control. (D) HFF cells were infected with WT virus or ADmUAPUL69 virus at an MOI of 0.5 PFU/cell. Forty-eight hours postinfection (hpi), cells were split onto coverslips. At 72 hpi, murine 3T3 cells were added to the coverslips and incubated for 4 h. Cycloheximide (50 μg/ml) was added to the cultures 30 min prior to and during the fusion process. One and a half hours after the fusion process, the cells were fixed with paraformaldehyde and stained with UL69 antibody and Hoechst stain. Virus-encoded green fluorescent protein (GFP) was used to aid in the identification of heterokaryons. Hoechst stain was used to distinguish HFF from 3T3 nuclei (3T3 nuclei display a punctate staining pattern, indicated with arrows). (E) Quantitative data for UL69 shuttling from three independent heterokaryon experiments. UL44 staining was included as a negative control for shuttling.