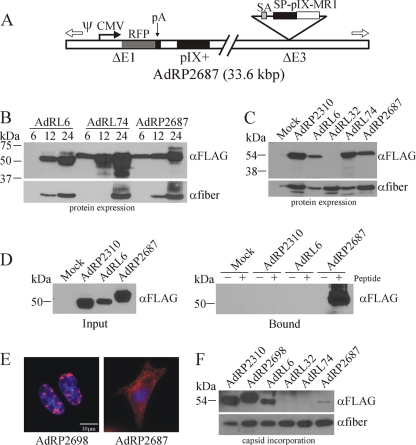
FIG. 4.
Coexpression of native pIX leads to low-level incorporation of pIX-MR1. (A) Structure of AdRP2687, which encodes red fluorescent protein (RFP) under the regulation of the human cytomegalovirus promoter (CMV) and bovine growth hormone polyadenylation sequence (pA), native pIX in its endogenous locus, and an SP-pIX-MR1-NLS expression cassette in the E3 region. Immediately 5′ of the pIX expression cassette is a splice acceptor (SA) derived from the Ad40 long fiber gene. (B) Kinetics of pIX-MR1 expression from viruses with different promoters. 293 cells were infected with the indicated viruses at an MOI of 1, and at 6, 12, or 24 h postinfection, crude cell extracts were prepared. At the end of the time course, samples were separated by SDS-PAGE and immunoblotted for the FLAG epitope on the pIX fusion proteins or for fiber. (C) 293 cells were infected with the indicated viruses, and 24 h later, crude protein extracts were prepared, separated by SDS-PAGE, and immunoblotted for the FLAG epitope on the pIX fusion proteins or for fiber. pIX-MR1 is efficiently expressed from AdRP2687. (D) 293 cells were infected with the indicated viruses, and 24 h later, the cells were lysed in a modified RIPA buffer and subjected to a pull-down assay with biotinylated EGFRvIII peptide as described in Materials and Methods. ER-targeted pIX-MR1 expressed from the E3 region of the virus is capable of binding the EGFRvIII epitope. (E) 293 cells were infected with AdRP2698 or AdRP2687, and 24 h later, the cells were fixed and analyzed by immunofluorescence for expression of the FLAG epitope tag present on the pIX fusion proteins. The resulting images were pseudocolored red to be consistent with images in Fig. 2. (F) Purified virions from the indicated viruses (2 × 108 particles) were separated by SDS-PAGE and immunoblotted for expression of the FLAG epitope on the pIX fusion proteins or for fiber. Coexpression of native pIX and SP-pIX-MR1 resulted in limited incorporation of the protein into the Ad capsid.