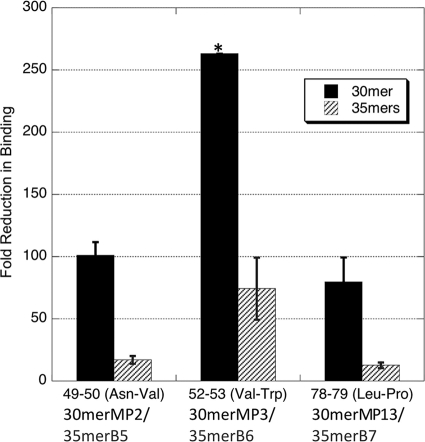
FIG. 5.
Comparison of effects of alanine mutations in the 30-mer and 35-mer peptides. The IC50s of the 30-mer and 35-mer peptides with the same double alanine mutations were compared to those of the corresponding peptides with the wild-type residues. The relative fold reductions in IC50s of the 30-mers and 35-mers were similar. Overall, the affinity reduction caused by mutations in the 30-mers was about four to seven times that caused by mutations in the 35-mers. The error bars were calculated from standard errors of IC50s of related peptides by error propagation. *, the standard error corresponding to residues 52 and 53 cannot be calculated for 30merMP3, because its IC50 cannot be determined accurately due to very loose binding of 30merMP3 and gH/gL.