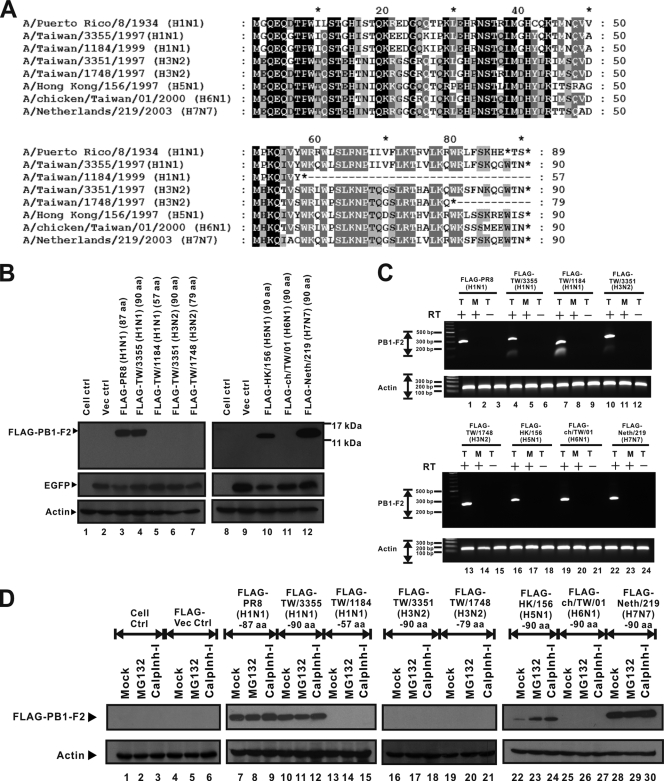
FIG. 1.
Sequence differentials and various expression levels of eight PB1-F2 variants. (A) Amino acid sequences of PB1-F2 variants that represent all influenza A virus subtypes available in this work. Sequence alignment and analysis were performed using BioEdit (version 7.0.5.2) and GeneDoc (version 2.7.000) software. Black shading, 100% identity; dark gray shading, 80% identity; pale gray shading, 60% identity. Sequence variations are caused by differences in protein lengths and amino acid sequences, especially in the C-terminal region. However, the amino acid sequence in the N-terminal region is more conserved than that in the C-terminal region. (B) FLAG-tagged PB1-F2 variant expression plasmids and the pEGFP-N2 vector were cotransfected into 293T cells. The EGFP expression level represents transfection efficiency, and the actin expression level represents the loading control level. Western blotting shows that expression of the FLAG-PR8 (H1N1) (87 aa) (lane 3), FLAG-TW/3355 (H1N1) (90 aa) (lane 4), FLAG-HK/156 (H5N1) (90 aa) (lane 10), and FLAG-Neth/219 (H7N7) (90 aa) (lane 12) PB1-F2 proteins was detectable. PB1-F2 protein expression by the other four PB1-F2 variants, from FLAG-TW/1184 (H1N1) (57 aa) (lane 5), FLAG-TW/3351 (H3N2) (90 aa) (lane 6), FLAG-TW/1748 (H3N2) (79 aa) (lane 7), and FLAG-ch/TW/01 (H6N1) (90 aa) (lane 11), was not detectable by Western blotting. ctrl, control. (C) FLAG-tagged PB1-F2 variant expression plasmids were transfected into 293T cells. At 24 h posttransfection, RNAs were extracted from transfected 293T cells. T, RNAs extracted from transfected cells; M, RNAs prepared from mock-transfected cells; +, RNAs that were transcribed by reverse transcriptase; −, RNAs that were not treated with reverse transcriptase before PCR. The level of expression of actin gene RNA is presented as the level for the loading control. Each transcript of the FLAG-tagged PB1-F2 expression plasmids was detectable by RT-PCR. (D) FLAG-tagged PB1-F2 expression plasmids were transfected into 293T cells. At 8 h posttransfection, MG132 and calpain inhibitor I (calplnh-I) were added to transfected 293T cells. At 16 h after treatment, total cell lysate was extracted and assayed by Western blotting. As shown in the Western blot, the proteins of FLAG-TW/1184 (H1N1) (57 aa) (lanes 13 to 15), FLAG-TW/3351 (H3N2) (90 aa) (lanes 16 to 18), FLAG-TW/1748 (H3N2) (79 aa) (lanes 19 to 21), and FLAG-ch/TW/01 (H6N1) (90 aa) (lanes 25 to 27) could not be rescued by proteasome inhibitors. However, the amount of PB1-F2 protein of FLAG-HK/156 (H5N1) (90 aa) (lanes 22 to 24) increased upon treatment with proteasome inhibitors.