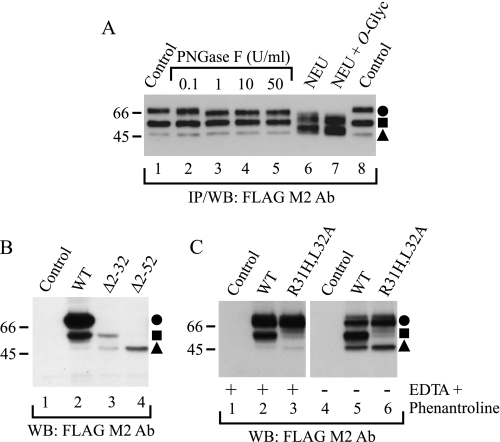
FIGURE 6.
Identification of hβ1AR cleavage sites. A, localization of cleavage sites in relation to N- and O-glycans. Stably transfected HEK293i cells were induced for 24 h, and DDM-solubilized receptors were purified with the anti-FLAG M2 antibody (Ab). EDTA and 1,10-phenanthroline were omitted from the homogenization buffer. Aliquots of the eluates were subjected to digestion with 0.1, 1, 10, or 50 units/ml PNGase F (lanes 2–5, respectively) or, alternatively, with 50 milliunits/ml neuraminidase (NEU; lane 6), or 50 milliunits/ml neuraminidase and 100 milliunits/ml O-glycosidase (O-glyc; lane 7) for 16 h at 30 °C. The control samples (lanes 1 and 8) contained buffer only. B, identification of the cleavage sites by means of receptor truncation mutants. C, site-directed mutagenesis of the major cleavage site at Arg31. Constructs for the wild-type hβ1AR, the truncation mutants hβ1ARΔ2–31 and hβ1ARΔ2–52, and the R31H,L32A mutant were transiently expressed for 24 h in Flp-In-293 cell, the control cells being transfected with vector DNA. Cells were homogenized in the absence or presence of EDTA and 1,10-phenanthroline, as indicated, and DDM-solubilized receptors were subjected to Western blotting (WB) using the anti-FLAG M2 antibody. The truncation mutants were expressed at a substantially lower level than the wild-type receptor (C), most likely because they lacked the HA signal sequence. WT, wild type; IP, immunoprecipitation. Symbols are as in Fig. 1.