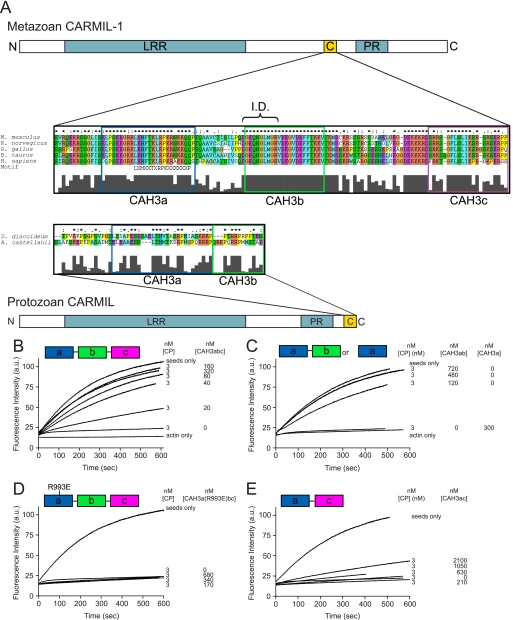
FIGURE 1.
The organization of CARMIL proteins and the conserved CAH3 domain and the identification of CAH3a/b as the minimal CAH3 domain-containing fragment of mCARMIL-1. A, domain organization of protozoan and metazoan CARMIL proteins. Both contain a leucine-rich repeat domain (blue), a proline-rich (PR) domain (blue), and a CAH3 domain. The CAH3 domain (gold), which is necessary and sufficient for the anti-CP activities of CARMIL, is N-terminal to the PR domain in metazoan CARMIL proteins and C-terminal to the PR domain in protozoan CARMIL proteins. Sequence alignments of the CAH3 domains from several species are also shown. These alignments reveal that metazoan CARMIL CAH3 domains can be subdivided into three conserved subdomains, CAH3a, CAH3b, and CAH3c, whereas protozoan CARMIL CAH3 domains contain only the CAH3a and CAH3b subdomains. The consensus CP-binding motif LXHXTXXRPKX6P (21) is indicated, as is the capping “interference domain” (I.D.) defined in this work. B–E, shown are actin polymerization assays using 10% pyrene-labeled G-actin, actin seeds, and the indicated concentrations of CP and the following CAH3 domain fragments of mCARMIL-1: CAH3a/b/c (B), CAH3a/b or CAH3a only (C), CAH3a(R993E)/b/c (D), or CAH3a/c (E). Actin polymerization was monitored by pyrene fluorescence. The CAH3a, CAH3b, and CAH3c subdomains are shaded green, pink, and blue, respectively.