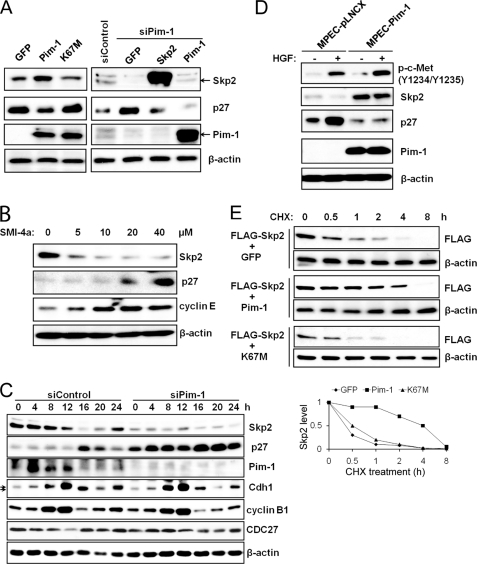
FIGURE 1.
Regulation of Skp2 protein levels by Pim-1. A, HeLa cells were transiently transfected with cDNAs encoding green fluorescent protein (GFP), Pim-1, kinase-dead Pim-1 (K67M), or a siRNA to Pim-1 together with GFP, or Skp2, or Pim-1, or a scrambled sequence. Forty-eight h after transfection, extracts of these cells were probed on Western blots with the listed antibodies. B, HeLa cells were treated with various concentrations of Pim kinase inhibitor SMI-4a for 16 h, extracts were prepared, and immunoblotting was carried out with the identified antibodies. C, HeLa cells were transfected with the indicated siRNA plasmids followed by a double-thymidine block treatment (see “Experimental Procedures”). Lysates were prepared at the indicated time points after release from the thymidine block and subjected to immunoblot analysis. Arrows indicate phosphorylated and unphosphorylated forms of Cdh1. D, mouse prostate epithelial cells (MPECs) stably transfected with a control vector (pLNCX) or a human Pim-1-expressing plasmid were treated with HGF (50 ng/ml) for 24 h followed by immunoblot analysis. E, 24 h after transfection with expression plasmids (time 0), HEK293T cells were incubated for the indicated times with cycloheximide (CHX, 100 μg/ml) followed by immunoblot analysis with FLAG or β-actin antibodies. Densitometric analysis was performed using ImageJ software to quantify the expression of Skp2. Skp2 band intensity was normalized to β-actin, then normalized to the t = 0 controls.