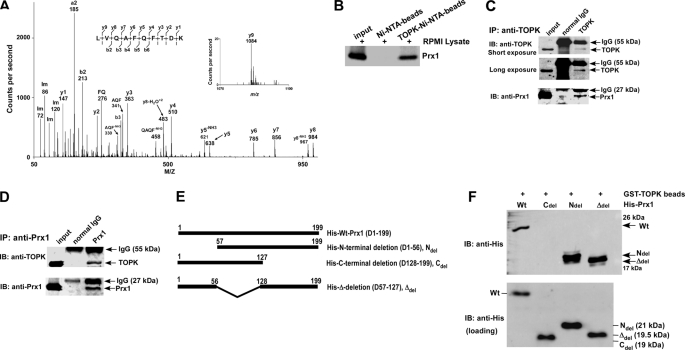
FIGURE 1.
TOPK interacts with Prx1 in vitro and ex vivo. A, tandem mass spectrum for the doubly charged ion from the peptide sequence LVQAFQFTDK (theoretical, monoisotopic neutral MW of 1195.62 Da), labeled with select experimental product ions, corresponding m/z values, and select ammonium ions (Im). The precursor mass error is 40 ppm. The experimentally determined diagnostic b- and y-type product ions with S/N >3 are shown on the peptide sequence above the spectrum (S/n = signal to noise). B, TOPK binding with Prx1 ex vivo. Lane 1: input, whole-cell lysate from RPMI7951 human melanoma cells; lane 2: negative control, whole-cell lysate from RPMI7951 cells precipitated with Ni-NTA-agarose beads; lane 3: whole-cell lysate from RPMI7951 cells precipitated with TOPK-Ni-NTA-agarose beads. TOPK and Prx1 binding was confirmed by immunoblotting with anti-Prx1. C and D, the endogenous TOPK-Prx1 complex was immunoprecipitated from RPMI7951 human melanoma cells with a TOPK (C) or a Prx1 (D) antibody, and TOPK or Prx1 were detected by Western blotting with a TOPK or Prx1 antibody. Precipitation with normal IgG served as a negative control. E, schematic diagrams for construction of Prx1 deletion mutants. Ndel (D1–56), Cdel (D128–199), or Δdel (D57–127) were each introduced into the pET46 Ek/LIC His fusion vector. F, Prx1 (Wt) and deletion mutants (Ndel, Cdel, or Δdel) purified from BL21 (bottom) were used for binding with GST-TOPK beads (top). Protein abundance were confirmed by Western blotting with an antibody against the His tag.