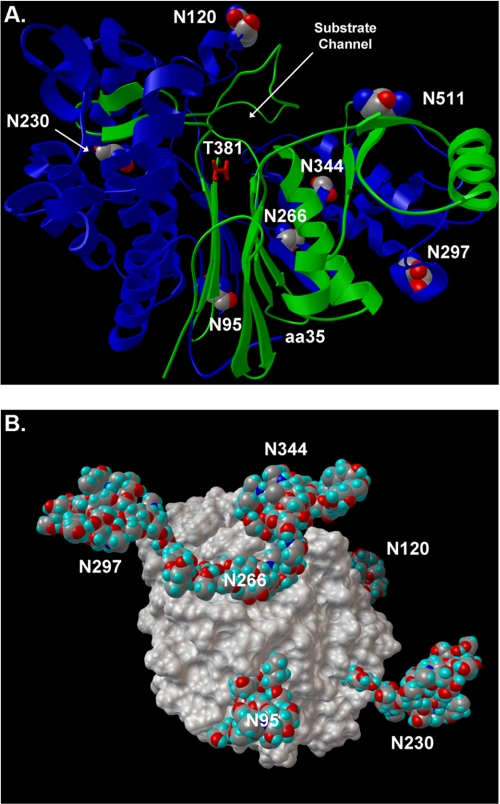
FIGURE 8.
Human GGT homology model. A, ribbon diagram of human GGT (large subunit, blue; small subunit, green). Glycosylated asparagine residues are highlighted with CPK atom coloring, and their positions in the amino acid sequence are indicated with white numbers. The position of the substrate channel and catalytic threonine (T381, red sticks) are shown. The homology model is based on the crystal structure of soluble GGT from E. coli. The first 34 amino acids of human GGT contain the transmembrane domain and do not have homology to E. coli GGT. The position of amino acid 35 (aa35) is shown, which defines the orientation of the enzyme on the cell surface. B, space-filling model of human renal GGT with the most abundant N-glycans identified at each site (Table 1). Rotational view of the renal heterodimeric enzyme, featuring a view from the intracellular perspective (i.e. base of transmembrane stalk). The Asn-511 glycosylation site is located on the distal surface of GGT and is obscured by the N-glycan at position Asn-344.