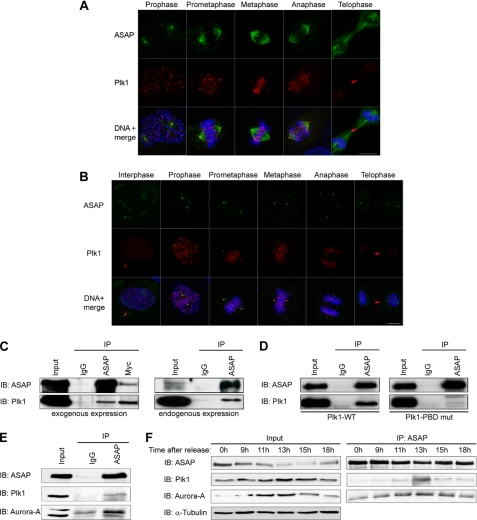
FIGURE 1.
ASAP and Plk1 co-localize at centrosomes and interact in vivo during mitosis. A and B, asynchronous U-2 OS cells were grown on glass coverslips and fixed in PAF/MTSB (A) or F/PHEM/methanol (B) (see “Materials and Methods”) and labeled with the polyclonal anti-ASAP (green), or the monoclonal anti-Plk1 antibodies (red) and stained with Hoechst 33258 (blue) without (A) or after (B) depolymerization of MTs by incubation on ice for 60 min. The different stages of the cell cycle are shown from prophase (A) or interphase (B) to telophase (scale bar, 10 μm). C, U-2 OS cell lysates were immunoprecipitated (IP) with rabbit IgG, rabbit anti-ASAP, or anti-Myc antibodies, and the precipitates were analyzed by immunoblotting (IB) with antibodies against ASAP or Plk1. Left, asynchronous cells were co-transfected with FLAG-ASAP and Myc-Plk1 (Input, 10% of protein extracts; immunoprecipitated with 200 μg of protein extracts). Right, endogenous proteins were co-immunoprecipitated from synchronized mitotic cells (see “Materials and Methods”) (Input, 10% of protein extracts; immunoprecipitated with 2 mg of protein extracts). A higher exposure is shown for Plk1 immunoblot. See supplemental Fig. S1A for uncropped gels. D, asynchronous U-2 OS cells were transfected with FLAG-ASAP and either Myc-Plk1-WT (left) or Myc-Plk1-PBD mutant (mut) (right) and immunoprecipitated with rabbit IgG or rabbit anti-ASAP antibody. Precipitates were analyzed by immunoblotting with antibodies against ASAP or Plk1. See supplemental Fig. S1B for uncropped gel. E, U-2 OS cells were transfected with FLAG-ASAP, and 24 h later, cell lysates were immunoprecipitated with rabbit IgG or rabbit anti-ASAP antibodies (Input, 10% of protein extracts immunoprecipitated with 200 μg of protein extracts). Precipitates were analyzed by immunoblotting with antibodies against ASAP, Plk1, or Aurora A. See supplemental Fig. S1C for uncropped gel. F, FLAG-ASAP-transfected cells were arrested at the G1/S boundary by thymidine block (2 mm for 24 h) and then released into fresh medium. Samples harvested at the indicated time points were analyzed by immunoblotting using antibodies against ASAP, Plk1, or Aurora A. Inputs are shown on the left and immunoprecipitates on the right. α-Tubulin was used as a loading control.