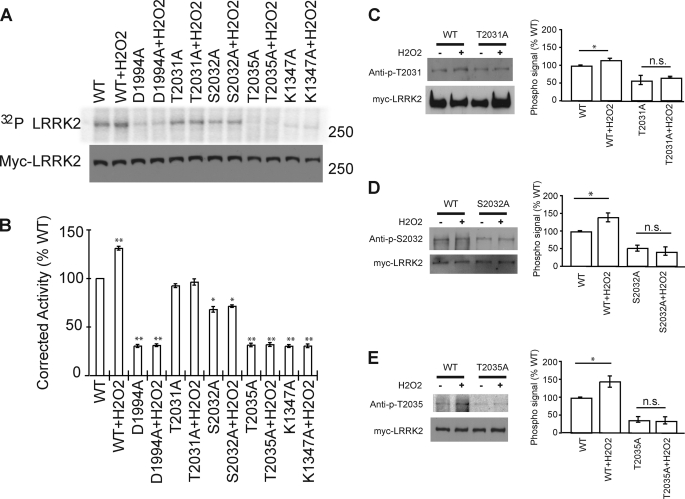
FIGURE 5.
Hydrogen peroxide (H2O2) activates LRRK2 kinase through the phosphorylation of all three phosphorylation sites (Thr-2031, Ser-2032, and Thr-2035). A, immunoblot and autoradiogram of autophosphorylated LRRK2, as resolved by SDS-PAGE. LRRK2 protein levels were determined by Western blot analysis, using anti-myc antibody. B, normalization of incorporated 32P compared with LRRK2 protein content. Data represent three independent experiments, in arbitrary units, where wild type (WT)-LRRK2 kinase activity is defined as 100%. Control bar represent mean ± S.E. *, p < 0.05; **, p < 0.01 compared with wild type LRRK2 kinase activity, assessed by a two-tailed one-sample Student's t test. C–E, Western blot using phospho-specific antibodies to detect the increasing phosphosignal of LRRK2 under the peroxide treatment. HEK-293 cells transfected with wild type or phospho-deficient mutant LRRK2 were treated with peroxide and immunoprecipitated by anti-myc antibody. Data represent five independent experiments, in arbitrary units, where wild type LRRK2 signal is defined as 100%. Control bar represent mean ± S.E. *, p < 0.05 compared with phosphorylation level of WT-LRRK2, assessed by a two-tailed one-sample Student's t test. n.s is nonsignificant, comparing the treated and untreated LRRK2 mutant samples, assessed by two tailed unpaired Students' t test.