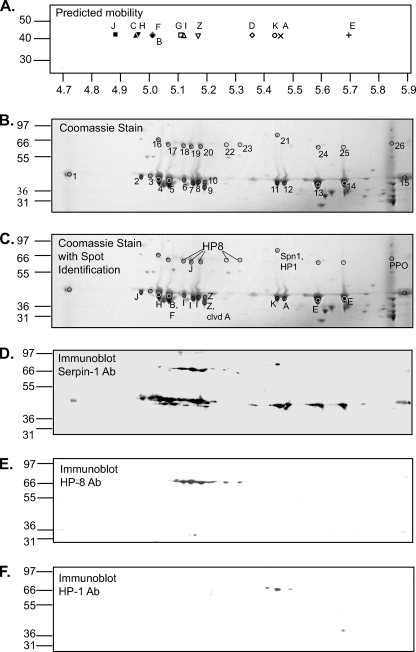
FIGURE 3.
Immunoaffinity-purified serpin-1 separated by isoelectric focusing and SDS-PAGE. A, the calculated isolectric points and masses of each serpin-1 isoform were used to predict separation after isoelectric focusing on a 4.7–5.9 pH range strip followed by SDS-PAGE. B, immunoaffinity-purified serpin-1 was separated by two-dimensional PAGE, using separation by IEF with a pH range of 4.7–5.9, followed by SDS-PAGE on a 4–12% acrylamide gradient gel. Coomassie Blue-stained spots corresponding to those from a duplicate gel detected by immunoblot analysis with serpin-1 antiserum (see panel D) were picked for trypsin digestion followed by MALDI-TOF and MALDI-TOF/TOF mass spectrometry analysis. Replicate gels were also analyzed after similar treatment with Glu-C and Lys-C/AspN (supplemental Fig. S3). C, summary of identification of serpin-1 isoforms and other proteins, based on mass spectrometry and immunoblot data. Panels D–F show immunoblot analysis of replicate two-dimensional PAGE samples using antisera to serpin-1 (D), HP8 (E), and HP1 (F).