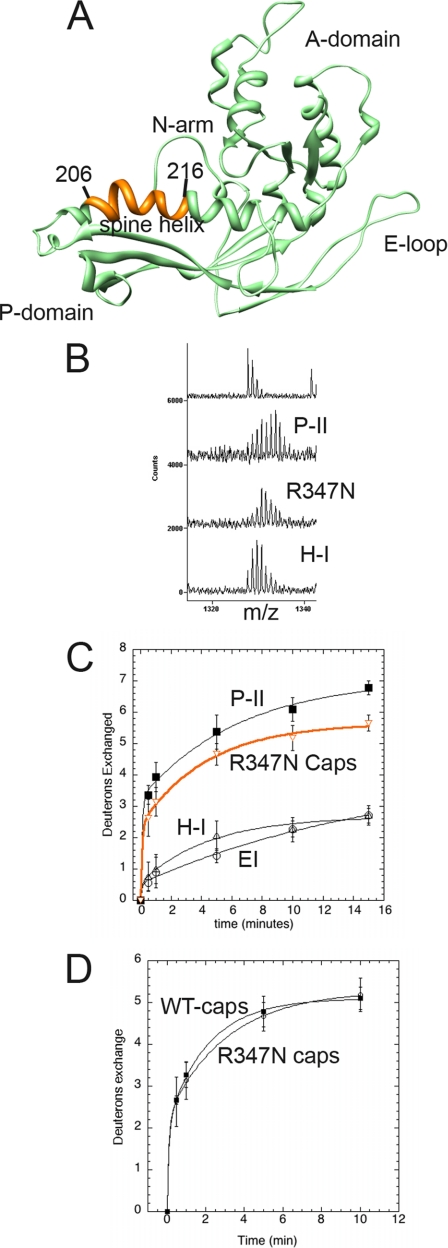
Fig. 8.
Solvent accessibility of R347N capsomer spine helix. A, subunit C of Prohead II is shown with the major domains labeled. Residues 206–216 of the spine helix are colored orange. B, mass envelopes for P-II and H-I particle forms as well as the R347N capsomers following 5 min of exchange. The top spectrum is non-deuterated P-II. C, H/2H exchange results of the residues colored orange are plotted for the R347N capsomers (orange curve) and compared with the solvent accessibility curves for the same fragment in the P-II capsid state, EI, and the nearly mature H-I capsid form. D, the solvent accessibility of the same spine helix fragment is shown for both the R347N capsomers and WT capsomers that were disassembled from the P-I state.