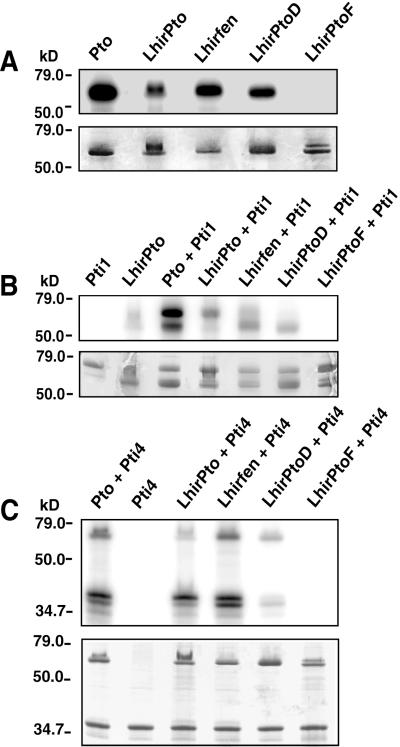
Figure 4.
Phosphorylation assays of the LhirPto proteins. (A) Pto and the LhirPto proteins were expressed in bacteria as GST fusions and purified on glutathione-agarose. Each protein (2 μg) was incubated in a kinase reaction with [γ-32P]ATP, separated by SDS/PAGE, and analyzed by autoradiography. (Upper) Autoradiogram of autophosphorylated LhirPto proteins. (Lower) Coomassie blue-stained gel. The locations of protein standards used to estimate molecular masses are indicated in kilodaltons (kD). (B) Assay to test phosphorylation of Pti1 by LhirPto proteins. GST-LhirPto proteins (2 μg) were incubated with 2 μg of purified kinase-deficient GST-Pti1-(K69N) in the presence of [γ-32P]ATP, separated by SDS/PAGE, and analyzed by autoradiography. (Upper) Autoradiogram of phosphorylated proteins. (Lower) Coomassie blue-stained gel. (C) Assay to test phosphorylation of Pti4 by LhirPto proteins. GST-LhirPto proteins (2 μg) were incubated with His-tagged Pti4 (2 μg) in the presence of [γ-32P]ATP, separated by SDS/PAGE, and analyzed by autoradiography. (Upper) Autoradiogram of phosphorylated proteins. (Lower) Coomassie blue-stained gel.