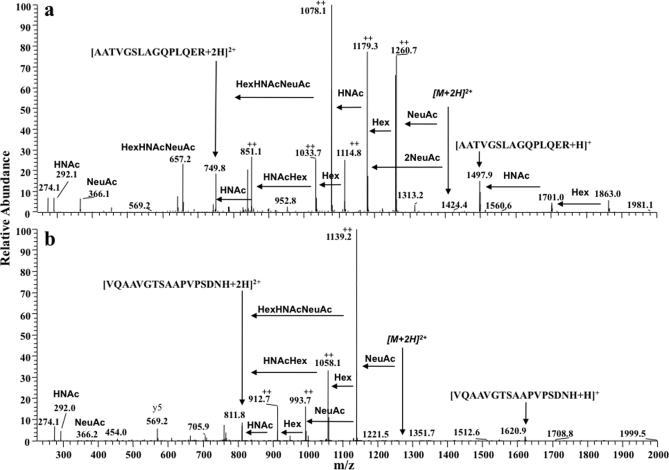
Fig. 3.
Ion trap MS/MS spectra of two distinct O-glycosylated peptides of apoE. Spectra of the most highly glycosylated peptides identified from each apoE glycosylation site are shown. Structures were determined from ion trap spectra even though the mass accuracy was ±m/z 0.4 and fragment ion charge states could not be accurately resolved. a, MS/MS analysis of an [M + 3H]3+ ion (m/z 937.08) present in cellular apoE. Glycan fragment ions (with respect to a double charged precursor) and losses are annotated, and the spectrum indicates glycopeptide apoE(192–206)-(HexNAc)2-Hex2-(NeuAc)2. b, ion trap MS/MS analysis of the [M + 3H]3+ ion (m/z 856.67) present in cellular apoE. The spectrum is of protonated apoE(283–299)-HexNAc-Hex-(NeuAc)2 and shows the same fragment ions observed with the spectrum acquired using the Q-Tof Ultima (Fig. 1b). The fragment ions indicated by ++ are assumed to be double charged. HNAc, HexNAc.