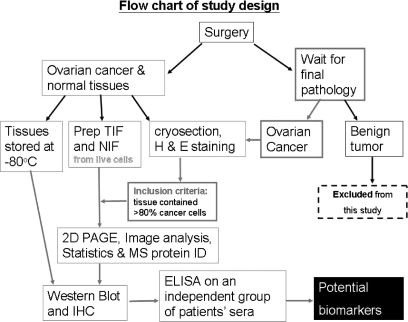
Fig. 1.
Study design for identifying potential serum biomarkers for human ovarian cancer. The TIF and NIF were prepared from enrolled cases. However, only those that passed the following criteria were further analyzed with two-dimensional PAGE, Western blot, and immunohistochemistry (the flows are marked with red arrows). The two criteria were: (i) pathological confirmation of ovarian cancer and (ii) that the majority (>80%) of the collected tissue were cancer cells. Prep, prepare; H & E, hematoxylin and eosin; MS protein ID, MS for protein identification; 2D, two-dimensional.