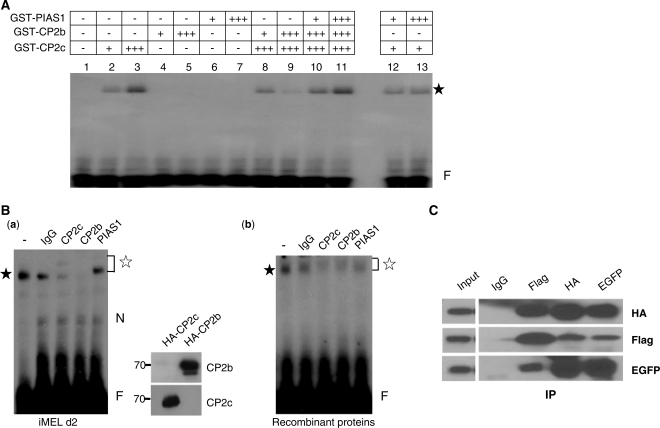
Figure 4.
PIAS1 enhances the DNA-binding ability of the CP2c/CP2b complex. (A) GST-PIAS1 and GST-CP2 isoforms purified from bacteria were used in EMSA. For EMSA, each protein, individually or in combination, was incubated with the 32P-labeled DNA probe for consensus CP2 binding sites derived from the mouse α-globin promoter, as indicated in ‘Materials and Methods’ section. An asterisk indicates the protein–DNA complex. A plus sign (+) indicates 0.2 µg protein. (B) MEL cell nuclear extracts at differentiation Day 2 (a) or purified bacterial GST-CBP proteins (CP2c/CP2b/PIAS1) (b) were used in supershift EMSA assays. Mock assay (lane 1) and assays in which 3 μg IgG, anti-CP2c, anti-CP2b or anti-PIAS1 antibody was added to the reaction mixtures shown in (A) (lanes 2–5). The closed and open asterisks indicate the specific protein–DNA and supershifted protein–DNA complexes, respectively. N and F represent the nonspecific and free probe bands, respectively. The specificities of anti-CP2b and CP2c antibodies were tested by western blotting [(a), right]. Custom peptide antibody was prepared using CP2b peptide (271-LTEMRLEPIIEDAVEHEQKKSSKRTLPADYGDSLAKRGS-309). The HA-CP2b or HA-CP2c expression vector was transfected into 293T cells, and total lysates were prepared. Proteins (10 μg) were analyzed by western blotting with anti-CP2b or anti-CP2c antibody. (C) 293T cells were transiently cotransfected with pcDNA3-FLAG-PIAS1, pcDNA3-HA-CP2c and pEGFPN1-CP2b vectors. Cell lysates prepared from these cells were immunoprecipitated using IgG, anti-FLAG, anti-HA or anti-EGFP antibody, and precipitates were analyzed in western blot assays using antibodies to EGFP, FLAG or HA.