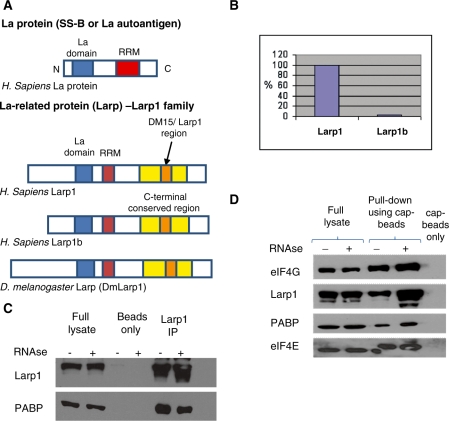
Figure 1.
(A) Larp proteins are conserved in metazoans and members of the Larp1 family contain an N-terminal La domain (similar to La proteins) and a C-terminal conserved or Larp1 region containing DM15 tandem repeat regions (33). There is a single ‘Larp’ gene in D. melanogaster [now termed DmLarp1 (27)] and two homologues in humans, Larp1 and Larp1b (previously termed Larp2) encoded by genes located at 5q33.2 and 4q28.2, respectively. Larp1 and Larp1b share 50 and 59% identity with Dm Larp1, respectively. There are two isoforms of Larp1 protein of 1019 and 1096 amino acids, respectively, and three isoforms of Larp1b protein of 914, 512 and 358 amino acids. (B) Of the two human Larp proteins, Larp1 is more abundant than Larp1b as shown by qPCR in HeLa cells (after normalization to housekeeping genes). The relative expression level of Larp1b (when standardized to 100% Larp1) was 2.5%, therefore Larp1 is 40-fold more abundant. (C) Immunoprecipitation, using antibodies to Larp1 and PABP and protein G sepharose beads showed an interaction between Larp1 and PABP, as demonstrated using western blotting of the immunoprecipitate. This interaction was not observed using beads alone (control) but was not disrupted following treatment with RNAse A. (D) Western blot of protein pull-down using 7-Methyl-GTP cap-binding sepharose beads demonstrates that Larp1 exists in complex with eIF4E. As expected, PABP and eIF4G were also ‘pulled down’ from the lysate using the cap beads in an association that was not disrupted after RNAse treatment. Additionally, loss of Larp1 (by RNAi) did not remove the interaction between either PABP and eIF4E or eIF4G and eIF4E (data not shown), suggesting these interactions are non-Larp1 dependent.