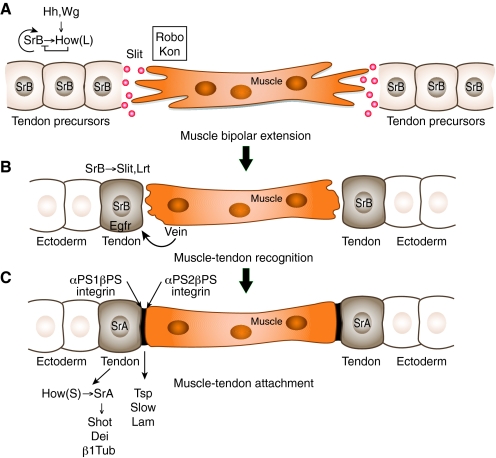
Fig. 1.
Different stages in Drosophila muscle-tendon interactions during embryogenesis. (A) In the first stage, tendon progenitors are defined in the ectoderm by the induction of StripeB (SrB) expression by the products of segment polarity genes, such as Hedgehog (Hh) and Wingless (Wg). StripeB expression is maintained at a low level as a result of post-transcriptional repression by the RNA-binding protein How(L). SrB regulates its own expression positively, as well as the expression of its inhibitor How(L). Tendon progenitors secrete Slit and provide initial cues for directing muscle bipolar migration. The muscle responds to Slit through Robo receptors. In addition, Kon-tiki (Kon) contributes to the migration of the muscles. (B) In a second step, the muscle signals to the tendon through epidermal growth factor receptor (Egfr) activation by means of the neuregulin-like secreted ligand Vein to initiate tendon differentiation and elevate SrB expression. SrB further induces the expression of Slit and Lrt; Lrt is required to arrest muscle migration. Tendon precursors that do not bind muscles lose SrB expression and become ectoderm cells. (C) In a third step, the myotendinous junction (black) is formed through muscle-specific αPS2βPS integrin association with the tendon-secreted ECM component Thrombospondin (Tsp) and its regulator Slow. Laminin (Lam) binds the αPS1βPS tendon-specific integrin. At this stage, How(S) is elevated in the cytoplasm of the tendon cell, promoting the expression of StripeA (SrA), which is essential to induce tendon terminal differentiation by induction of Short stop (Shot), Delilah (Dei), and β1 Tubulin (β1Tub).