Abstract
Objective
Studies in obesity have implicated adipocytokines in the development of insulin resistance, which in turn may lead to accelerated aging. In this study, we determined associations of chromosomal telomere length (TL) to markers of obesity and insulin resistance in middle-aged adult male and female Arabs with and without diabetes mellitus type 2 (DMT2).
Design and methods
One hundred and ninety-three non-diabetic and DMT2 subjects without complications (97 males and 96 females) participated in this cross-sectional study. Clinical data, as well as fasting blood samples, were collected. Serum glucose and lipid profile were determined using routine laboratory methods. Serum insulin, leptin, adiponectin, resistin, tumor necrosis factor-α, and PAI-1 were quantified using customized multiplex assay kits. High sensitive C-reactive protein (hsCRP) and angiotensin II (ANG II) were measured using ELISAs. Circulating leukocyte TL was examined by quantitative real-time PCR.
Results
Circulating chromosomal leukocyte TL had significant inverse associations with body mass index (BMI), systolic blood pressure, fasting insulin, homeostasis model assessment of insulin resistance (HOMA-IR), low-density lipoprotein (LDL)- and total cholesterol, ANG II and hsCRP levels. Adiponectin, BMI, systolic blood pressure, and LDL cholesterol predicted 47% of the variance in TL (P<0.0001). HOMA-IR was the most significant predictor for TL in males, explaining 35% of the variance (P=0.01). In females, adiponectin accounted for 28% of the variance in TL (P=0.01).
Conclusion
Obesity and insulin resistance are associated with chromosomal TL among adult Arabs. Evidence of causal relations needs further investigation. The positive association of adiponectin to TL has clinical implications as to the possible protective effects of this hormone from accelerated aging.
Introduction
The prevalence of obesity has increased in many human populations regardless of age, gender, ethnicity, and socioeconomic status (1). Developing nations, such as Saudi Arabia, are not immune to this global plague, with an alarming prevalence of 35 and 37% in obese and overweight adult citizens respectively (2). Furthermore, the indigenous Saudi population seems to have a special genetic predisposition to develop diabetes mellitus type 2 (DMT2) (3).
Recent studies in obesity highlight the importance of several adipose-derived hormones, also known as adipocytokines, in the development of insulin resistance (4, 5). Adipocytokines have physiological effects on a multitude of metabolic pathways and are altered in the presence of increased fat mass, particularly abdominal obesity, e.g. elevation of certain adipocytokines, such as leptin, and decrease of others, such as adiponectin (6).
Cardiometabolic complications of obesity progressively increase with age, with obese individuals presenting accelerated chronic non-communicable disease morbidity and mortality (7). In recent years, it has become possible to correlate an individual's age with the length of the telomeres in his/her mature circulating leukocytes. Telomeres are tandem repeats of the DNA sequence TTAGGG extending over 6–15 kb at the end of eukaryotic chromosomes, necessary for both successful DNA replication and maintenance of chromosomal integrity (8). Telomere length (TL) declines with age in mature endothelial cells also, and is thought to contribute to endothelial dysfunction and atherogenesis (9). Recently, chromosomal TL was associated negatively with an increase in the risk for the development of several chronic pathologies, such as coronary artery disease (CAD) (10), obesity (11), and insulin resistance (12). In obese men, shortened leukocyte TL was a powerful marker of increased carotid artery intimal medial thickness (13).
To date, no studies have been carried out in high-risk ethnic groups, such as the Saudi Arabians. This population has distinct diet and activity patterns and a homogeneous genetic background. This cross-sectional study assessed circulating leukocyte TL and determined whether its variations influence biomarkers of obesity and insulin resistance, including a host of adipocytokines and inflammatory markers, in a cohort of middle-aged non-diabetic and type 2 diabetic Saudi Arabs.
Patients and methods
Clinical subjects
This cross-sectional study was carried out at the Diabetes and Endocrinology Research Laboratory of the King Saud University, Riyadh, KSA. A total of 193 ambulatory and asymptomatic participants (97 males and 96 females, aged 18–66 years, with varying body mass index (BMI) (lean–obese)) were recruited. Only patients who were on medications for diabetes and hypertension without complications (e.g. diabetic complications, CAD, and liver or kidney failure) were included to avoid selection bias. Patients were asked to complete general questionnaires, which included detailed medical history, diet, and physical activity. Written informed consent was also obtained, prior to inclusion in the study. Ethical approval was obtained from the ethics committee of the College of Medicine Research Center in King Khalid University Hospital, Riyadh, KSA.
Anthropometric measurements
Anthropometric data were collected by a designated research nurse and physician, as part of an ongoing research program ascertaining height (to the nearest 0.5 cm), weight (to the nearest 0.1 kg), and waist and hip circumferences (measured using a standardized measuring tape in cm), in addition to systolic and diastolic blood pressure measurements. BMI was calculated as kg/m2. Obesity was defined as having a BMI of ≥30 kg/m2, while overweight was defined as a BMI of >25 but <30 kg/m2.
Biochemical measurements
Morning fasting blood samples were collected from all subjects on an assigned date. Serum was obtained by centrifugation and transported to the laboratory. Serum glucose and lipid profile were determined using routine laboratory methods. Serum insulin, leptin, adiponectin, resistin, tumor necrosis factor (TNF)-α, and PAI-1 (SERPINE1 as listed in the Hugo Database) were quantified using multiplex assay kits that utilize fluorescent microbead technology, allowing simultaneous quantification of several target proteins within a single serum sample of 50–100 μl (14). These included pre-mixed and fully customized panels that utilize the Luminex xMAP Technology platform (Luminex corporation, Austin, TX, USA). For the parameters measured using the multiplex assay, the intra-assay variation was 1.4–7.9% and inter-assay variation of <21%. Minimum detectable concentrations (MDC) were as follows: insulin, 50.9 pg/ml; leptin, 85.4 pg/ml; adiponectin, 145.4 pg/ml; resistin, 6.7 pg/ml; TNF-α, 0.14 pg/ml; and PAI-1, 1.3 pg/ml. High sensitive C-reactive protein (hsCRP) was determined using ELISA kit Bensheim, (Immunodiagnostik AG, Germany) with an intra-assay variation of 5.5–6.0% and inter-assay variation of 11.6–13.8%. Angiotensin II (ANG II) was quantified using fluorescent-based non-radioactive immunoassay (MDC 13 pg/ml; linear range 13–240 pg/ml; Phoenix Pharmaceuticals, Burlingame, CA, USA). All fasting samples fell within the detection range except for one sample in ANG II analysis, which was below the detection limit (ANG II=10 pg/ml). HOMA-IR was calculated as fasting insulin (μU/ml)×fasting glucose (mmol/l)/22.5.
TL analysis
For TL determination, DNA was extracted from leukocytes isolated from whole blood. TL was examined by quantitative real-time PCR utilizing IQ cylinder. The assay involved comparing the abundance of telomere DNA to an internal reference gene of invariant copy number for each sample and by further comparison of normalized value between DNAs of different sources. By including DNA samples of known TL, the procedure was calibrated to estimate actual TL. For accurate quantification, the efficiency of the PCR (i.e. the actual fold increase in amplicon accumulation for each round of amplification) was determined by constructing a standard curve, and the samples assayed contained an amount of target that was within the region of the standard curve for which the efficiency could be accurately determined.
Two reference DNA samples (MRC5 and KE27) were used to construct standard curves of amplifications using GAPDH (fixed copy number reference gene) and telomere primer pairs; 1.68-fold serial dilutions were made for each DNA covering a range of 7.4–0.93 ng/μl. In total, 10 μl aliquots of the diluted reference DNAs were dispensed to each of four replicate wells for each dilution, giving final quantities in the range of 74–9.3 μl DNA per well. In total, 15 μl PCR cocktail containing 12.5 μl Taqman mastermix and 5 pmol each primer were added to each well, and plates were cycled 40 times at 95 °C for 15 s and 56 °C for 60 s. Plots of log [10] template quantity versus cycle threshold (Ct) showed linear relations across the entire dilution series for both primer pairs tested against both reference DNA samples. The slopes of the graphs were used to calculate average efficiency of amplification values for both primer sets.
Sample telomere assay
Test DNA samples were diluted to concentrations within the linear standard curve ranges and amplified under the same conditions as for the reference DNAs for both GAPDH and telomere primer pairs, employing four replicates for each sample. Values were normalized for each sample against the GAPDH signal by subtracting the GAPDH Ct value from the corresponding telomere Ct value (ΔCt values). The efficiency of amplification values derived from the standard curves was used to calculate the differences in abundance of the telomere amplicons for each amplicon, and these were corrected by calibration against the known lengths of the telomeres in the reference DNAs (determined by terminal restriction fragment length analysis reported earlier). Final values, expressed as length of telomere in bps, were calculated relative to the known standard lengths.
Power calculations and data analyses
Power calculations were undertaken for TL analysis on available current literature examining patients with DMT2 (15). A total sample size of 40 between two groups would give 80% power at the 5% level to detect a 1 s.d. difference between group means. Frequencies were presented as percentage, and continuous variables that assume normality were presented as mean±s.d., while medians (inter-quartile range) were shown for non-normal continuous variables. Independent Student's t-test was used to compare gender differences for normal parameters and χ2-test was used for frequencies. For non-normal parameters, Mann–Whitney U test was utilized. Metabolic parameters such as insulin, triglycerides, leptin, adiponectin, resistin, TNF-α, ANG II, and hsCRP were log transformed prior to Pearson correlation and regression analysis using TL as a dependent variable. To determine significant predictors of TL, stepwise linear regression analysis was performed. Independent variables entered were age, BMI, systolic and diastolic blood pressure, waist and hip circumferences, and all the metabolic parameters measured, while TL was used as a dependent variable. Significance was set at P<0.05. All statistical analyses were conducted using SPSS version 11.5 (Chicago, IL, USA).
Results
Gender differences in baseline characteristics
Men had elevated systolic and diastolic blood pressure compared with women of similar age range (P values 0.01 and 0.01 respectively). Women, on the other hand, had a significantly higher prevalence of obesity than men (P=0.001). Prevalence of DMT2 was similar in the two genders (P=0.07). In the glycemic profile, males had higher serum insulin levels than females (P=0.03), while both glucose and HOMA-IR values were similar to those of women. Triglyceride levels were higher in males than in females (P=0.01), while women had higher high-density lipoprotein (HDL) cholesterol levels than men at borderline significance (P=0.05). Finally, women had significantly higher levels of circulating leptin and adiponectin, as opposed to males, as expected (P values <0.001 and 0.03 respectively). Levels of resistin, TNF-α, aPAI-1, ANG II, hsCRP, and mean TLs were not different between the genders (Table 1).
Table 1.
Clinical characteristics, glycemic, lipid, and metabolic profiles of male and female subjects studied. Data is presented as n (%) for frequencies and mean±s.d. for normal continuous variables.
| Males | Females | P value | |
|---|---|---|---|
| n | 97 | 96 | |
| Clinical characteristics | |||
| Obese (n (%)) | 25 (25.8) | 44 (45.8) | 0.001 |
| Type 2 DM (n (%)) | 25 (25.8) | 29 (30.2) | 0.07 |
| Age (years) | 41.9±10.3 | 39.2±11.0 | 0.11 |
| BMI (kg/m2) | 27.1±4.9 | 29.6±6.2 | 0.004 |
| Systolic BP (mmHg) | 123.9±14.2 | 118.2±14.1 | 0.01 |
| Diastolic BP (mmHg) | 80.6±7.6 | 76.9±10.0 | 0.01 |
| Waist circumference (cm) | 92.6±17.1 | 87.6±17.6 | 0.06 |
| Hip circumference (cm) | 100.5±17.4 | 100.6±18.2 | 0.99 |
| Glycemic profile | |||
| Glucose (mmol/l) | 6.5±3.5 | 7.5±4.1 | 0.09 |
| Insulin (IU/ml)a | 8.2 (4.9–13.7) | 6.8 (4.6–9.2) | 0.03 |
| HOMA-IRa | 2.4 (1.5–3.8) | 2.1 (1.2–5.3) | 0.97 |
| Lipid profile | |||
| Triglycerides (mmol/l)a | 1.5 (1.0–2.3) | 1.1 (0.9–1.8) | 0.01 |
| Total cholesterol (mmol/l) | 5.2±1.2 | 4.9±1.1 | 0.15 |
| LDL cholesterol (mmol/l) | 3.4±0.9 | 3.3±1.0 | 0.54 |
| HDL cholesterol (mmol/l) | 0.84±0.2 | 0.92±0.2 | 0.05 |
| Metabolic profile | |||
| Leptin (ng/ml)a | 5.9 (2.7–12.7) | 25.9 (12.6–37.8) | <0.001 |
| Adiponectin (μg/ml)a | 10.8 (6.3–16.2) | 12.5 (8.2–18.3) | 0.03 |
| Resistin (ng/ml)a | 16.3 (12.6–24.2) | 18.6 (13.8–24.9) | 0.19 |
| TNF-α (pg/ml)a | 3.1 (2.3–4.7) | 3.6 (2.3–5.1) | 0.36 |
| aPAI-1 (pg/ml)a | 6.7 (2.1–18.7) | 9.2 (2.3–27.2) | 0.12 |
| ANG II (ng/ml)a | 0.6 (0.4–0.8) | 0.5 (0.4–0.7) | 0.28 |
| C-reactive protein (μg/ml)a | 2.8 (0.8–5.1) | 2.9 (1.2–8.4) | 0.3 |
| Telomere length (kb) | 5.4±1.6 | 5.1±1.6 | 0.41 |
Denotes continuous variables with non-Gaussian distribution and is presented as median (inter-quartile range); P value is significant at <0.05.
Associations of TL
The various cross-sectional associations of TL in men and women as well as all subjects combined are shown in Table 2. Considering all subjects, TL had significant inverse associations with BMI, insulin, HOMA-IR, low-density lipoprotein (LDL) cholesterol, and hsCRP levels. Adiponectin had a positive significant association with TL regardless of gender (P=0.04). Among men, TL had significant inverse correlations with waist circumference, insulin, LDL- and total cholesterol, and hsCRP. Moreover, HOMA-IR had a strong inverse correlation with TL in males (P<0.0001). In women, BMI, ANG II, and hsCRP had significant inverse associations with TL. A significant positive association of adiponectin levels to TL was observed in women (Table 3).
Table 2.
Correlations of telomere length to various clinical and metabolic parameters. Data is presented as coefficients.
| Parameter | Males | Females | All |
|---|---|---|---|
| Age (years) | 0.07 | −0.12 | 0.002 |
| BMI (kg/m2) | −0.25* | −0.26* | −0.27* |
| Systolic BP (mmHg) | 0.002 | −0.17 | −0.11 |
| Diastolic BP (mmHg) | 0.07 | −0.19 | −0.12 |
| Waist circumference (cm) | −0.26* | 0.05 | −0.047 |
| Hip circumference (cm) | −0.14 | 0.03 | −0.03 |
| Glucose (mmol/l) | −0.19 | 0.09 | 0.01 |
| Insulin (IU/ml) | −0.46† | 0.03 | −0.20* |
| HOMA-IR | −0.61† | 0.07 | −0.29† |
| Triglycerides (mmol/l) | −0.13 | −0.02 | −0.001 |
| Total cholesterol (mmol/l) | −0.23* | −0.10 | −0.15 |
| LDL cholesterol (mmol/l) | −0.33† | −0.15 | −0.21* |
| HDL cholesterol (mmol/l) | 0.23 | 0.09 | 0.12 |
| Leptin (ng/ml) | −0.14 | −0.20 | −0.16 |
| Adiponectin (μg/ml) | 0.16 | 0.26* | 0.20* |
| Resistin (ng/ml) | −0.05 | 0.11 | 0.03 |
| TNF-α (pg/ml) | 0.03 | −0.15 | −0.09 |
| aPAI-1 (pg/ml) | 0.16 | −0.16 | −0.03 |
| ANG II (ng/ml) | −0.22 | −0.26* | −0.22 |
| C-reactive protein (μg/ml) | −0.37† | −0.39† | −0.31† |
*P value is significant at 0.05 level; †P value is significant at 0.01 level.
Table 3.
Stepwise linear regression analysis using telomere length as a dependent variable and all parameters measured as independent variables.
| Gender | Predictor | β | s.e.m. | Adjusted R2 | P value |
|---|---|---|---|---|---|
| Male | HOMA-IR | −0.29 | 0.1 | 0.35 | 0.01 |
| Female | Adiponectin | 0.07 | 0.02 | 0.28 | 0.01 |
| All | Adiponectin | 0.08 | 0.02 | 0.47 | <0.0001 |
| BMI | −0.15 | 0.04 | |||
| Systolic BP | 0.05 | 0.02 | |||
| LDL cholesterol | −0.52 | 0.25 |
Independent variables entered were age, BMI, systolic and diastolic blood pressure, waist and hip circumferences, glucose, insulin, HOMA-IR, HDL-, LDL-, and total cholesterol, triglycerides, leptin, adiponectin, resistin, TNF-α, aPAI-1, ANG II, and CRP.
Predictors of TL
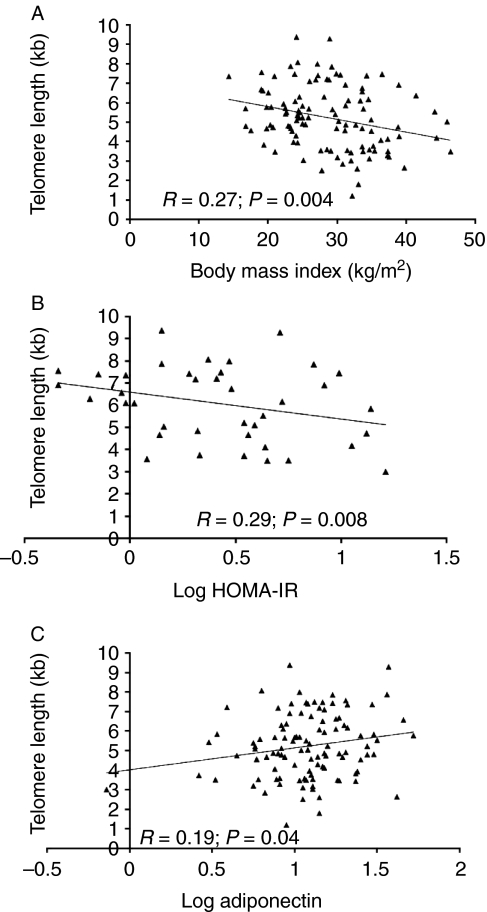
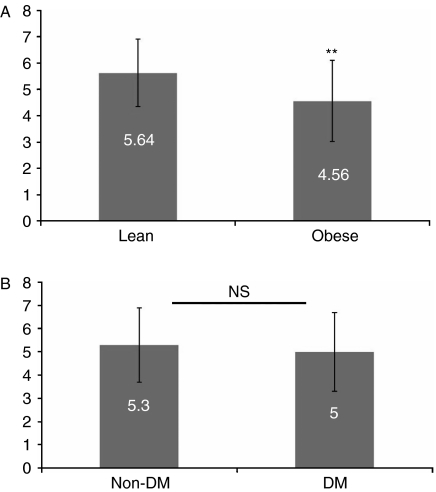
In all subjects, adiponectin, BMI, systolic blood pressure, and LDL cholesterol predicted 47% of the variance in TL (P<0.0001). Figure 1 shows the linear relations of TL to selected parameters, such as BMI, HOMA-IR, and adiponectin. A significant negative correlation of TL to hsCRP levels in all subjects (P=0.009) was noted, which, however, was lost after controlling for other confounders. In males, HOMA-IR was a significant predictor for TL, explaining 35% of variance (P=0.01). β value for HOMA-IR indicated that every 0.29 unit decrease corresponds to a 1 unit increase in TL. In females, a 0.07 unit increase in adiponectin corresponded to a 1 unit increase in TL, and this accounted for 28% of the variance in TL (P=0.01). Figure 2 shows the significantly shorter TL among obese subjects and the non-significant difference among those with and without DMT2.
Figure 1.
Linear regression plots between chromosomal telomere length and indices of obesity and insulin resistance: (A) TL versus BMI (R=−0.27; P=0.004); (B) TL versus log HOMA-IR (R=−0.29; P=0.008); (C) TL versus log adiponectin (R=0.19; P=0.04).
Figure 2.
Telomere length in lean and obese subjects (A) and non-diabetics and diabetics (B). **P=0.001; NS, non-significant.
Discussion
Adiponectin was positively associated with TL, while BMI as well as insulin resistance inversely influenced TL in middle-aged adults, even after controlling for other clinical and metabolic confounders. Hypoadiponectinemia is a good marker of insulin resistance and an independent risk factor for DMT2 and CAD (16). As an adipocytokine with insulin-sensitizing and anti-inflammatory properties, this hormone has protective effects against metabolic abnormalities that accelerate aging. In obese rats, adiponectin reversed endothelial dysfunction by increasing nitric oxide production by eNOS phosphorylation, and by decreasing nitric oxide inactivation through blocking superoxide production (17). Among patients with Hutchinson–Gilford Progeria syndrome, an age-related decrease in circulating adiponectin was coupled with a striking progressive loss of functional subcutaneous adipose tissue and was associated with premature atherosclerosis (18). Furthermore, anti-proliferative effects of adiponectin on MCF7 breast cancer cells were demonstrated in vitro (19), while apoptotic effects were noted when treatment adiponectin was extended (20). Furthermore, vascular calcification seen in atherosclerotic lesions is a common consequence of aging (21), while adiponectin antagonizes the stimulatory effect of TNF-α on vascular smooth muscle calcification by restoration of the AMPK-dependent Gas6-mediated survival pathway (22). The significant positive association of adiponectin to TL in this study suggests that adiponectin may be an anti-aging agent by way of improving insulin sensitivity, decreasing inflammation and cell oxidative function, and reversing endothelial dysfunction.
In our study, BMI and insulin resistance were associated with telomere loss, possibly explained as a result of cumulative psychological, metabolic, inflammatory, and oxidative stress leading to accelerated physiological aging (23, 24). Indeed, the presence of chronic psychological stress among adults related to the fast-paced modern lifestyle significantly contributes to obesity- and dysmetabolic syndrome-related aging (24–27). Chronic psychological stress may also lead to overeating and co-elevation of cortisol and insulin, causing accumulation of visceral fat overtime, translating into metabolic and inflammatory stress (25). The elevated hsCRP and ANG II levels in our subjects, indices of inflammatory and physiologic stress respectively, were both inversely correlated with TL. Thus, stress-related dysmetabolic and pro-inflammatory biochemical environment appears to be conducive to several cell aging mechanisms, ultimately leading to TL shortening and hence, cell senescence (27).
Increased circulation of inflammatory cytokines may also stimulate leukocyte turnover and mitochondrial activity with elevated production of reactive oxygen species (ROS) respectively, causing replicative senescence and damaging the telomeres of our patients (18). The inverse association of hsCRP to TL is in line with the findings of Farzaneh-Far et al., (28) and supports the hypothesis that systemic inflammation promotes both atherogenesis and telomere attrition. The association of ANG II to TL in this study, on the other hand, further strengthens the theory that ANG II directly contributes to cellular aging. A recent study demonstrated that ANG II induced ROS-mediated DNA damage resulting in accelerated biological aging of human vascular smooth muscle cells also via two mechanisms: first, through acute stress-induced telomere damage, and second, by accelerating replicative senescence and hence, telomere attrition (29). Chronic administration of ANG II receptor antagonist was shown to reset the hypothalamic–pituitary–adrenal axis, thereby improving the effect and mitigating the metabolic stress of patients with DMT2 (30).
Our findings contradict the study of Diaz et al. (31) who found no linear associations between measures of obesity and TL. Several factors could have attributed to this. First is the homogeneity of the population used in our study, as opposed to the racially and ethnically diverse cohort used in the Diaz study. The inclusion of diabetic subjects in this cohort, on the other hand, might have also influenced the differences in the result of the two studies.
The significant negative association of total cholesterol and LDL cholesterol to TL is also worthy to note. Elevated cholesterol levels are atherogenic and can produce repeated mechanical, hemodynamic, and/or immunological injury and, as such, may cause augmented cell turnover and increased production of ROS in certain cells (32). From this premise, it can be suggested that the link between cholesterol levels and TL is secondary to increased cell damage and turnover, which in turn amplifies cell aging by bringing cells to their maximum replicative capacity, translating to shortened TL. It could also tie in with the age-related innate immune pathway activation in adipose tissue and its link to subclinical chronic inflammation.
The seemingly paradoxical lack of association between TL and age is most likely because the bulk of the subjects studied were middle aged. This finding is in accordance with other studies and disputes the unconditional use of peripheral blood monocyte TL as a biomarker for aging due to telomere instability (33) and negative feedback regulation (34). Cross-sectional TL at a single age point reflects genetic background and cumulative lifetime burdens of environmental stress exposures (34), but the large inter-individual variation and unmeasured confounding factors, such as baseline TL and differences of somatic stem cell telomerase activity among subjects, may all account for the ‘unexplained’ lack of association. Male gender has been proposed to be an independent predictor for increased telomere attrition (34, 35), and the gender difference does not supersede other findings confirming an association between insulin resistance and telomere attrition in both genders (12).
In short, obesity and insulin resistance are associated with measurable changes in a multitude of potential contributing factors, including adipocytokines and inflammatory mediators, which may shorten TL and accelerate biological aging. Although the precise role of telomere shortening has yet to be elucidated, it is now clear that adiposity and insulin resistance collectively lead to accelerated aging that is associated with development and progression of chronic non-communicable diseases. The significant association of adiponectin to biological senescence has clinical implications as to its potential protective effects in slowing down physiologic aging, possibly by means of improving insulin sensitivity, and reducing systemic inflammation, ultimately mitigating endothelial dysfunction and development of atherosclerosis. Prospective, interventional studies are needed to test this hypothesis.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by King Abdul-Aziz City for Science and Technology (KACST; research project no. APR-26-046), Riyadh, Kingdom of Saudi Arabia.
Acknowledgements
The authors wish to thank the British Heart Foundation for funding an Intermediate Fellowship for Dr Alison Harte, as well as Research Council, UK, for funding Dr Tripathi in this work.
References
- World Health Organization 2003 Global Strategy on diet, physical activity and health, Available online: http://www.who.int/nut/obs.htm
- Al-Nozha MM, Al-Mazrou YY, Al-Maatouq MA, Arafah MR, Khalil MZ, Khan NB, Al-Marzouki K, Abdullah MA, Al-Khadra AH, Al-Harthi SS, Al-Shahid MS, Al-Mobeireek A, Nouh MS. Obesity in Saudi Arabia. Saudi Medical Journal. 2005;26:824–829. [PubMed] [Google Scholar]
- Elhadd TA, Al-Amoudi AA, Alzahrani AS. Epidemiology, clinical and complications profile of diabetes in Saudi Arabia: a review. Annals of Saudi Medicine. 2007;27:241–250. doi: 10.4103/0256-4947.51484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizmecioglu FM, Etiler N, Ergen A, Gormus U, Keser A, Hekim N, Hamzaoglu O, Hatun S. Association of adiponectin, resistin, high sensitive CRP level with the metabolic syndrome in childhood and adolescence. Experimental and Clinical Endocrinology and Diabetes. 2009;117:622–627. doi: 10.1055/s-0028-1112151. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Aeberli I. Dietary determinants of subclinical inflammation, dyslipidemia and components of the metabolic syndrome in overweight children: a review. International Journal of Obesity. 2009;32:S11–S18. doi: 10.1038/ijo.2008.202. [DOI] [PubMed] [Google Scholar]
- Al-Daghri NM, Al-Attas O, Al-Rubeaan K, Sallam R. Adipocytokine profile of type 2 diabetics in metabolic syndrome as defined by various criteria. Diabetes/Metabolism Research and Reviews. 2009;24:52–58. doi: 10.1002/dmrr.763. [DOI] [PubMed] [Google Scholar]
- Brouilette SW, Whitaker A, Stevens SE, van der Harst P, Goodall AH, Samani NJ. Telomere length is shorter in healthy offspring in subjects with coronary artery disease: support for the telomere hypothesis. Heart. 2008;94:422–425. doi: 10.1136/hrt.2007.139675. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Burkle A, Kirkwood TB. Stress, DNA damage and ageing: an integrative approach. Experimental Gerontology. 2001;36:1049–1062. doi: 10.1016/S0531-5565(01)00111-5. [DOI] [PubMed] [Google Scholar]
- Kushner EJ, Van Guilder GP, Maceneaney OJ, Cech JN, Stauffer BL, DeSouza CA. Aging and endothelial progenitor cell telomere length in healthy men. Clinical Chemistry and Laboratory Medicine. 2009;47:47–50. doi: 10.1515/CCLM.2009.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- Nordfjall K, Eliasson M, Stegmayr B, Lundin S, Roos G, Nilsson PM. Increased abdominal obesity, adverse psychosocial factors and shorter telomere length in subjects reporting early ageing: the MONICA Northern Sweden Study. Scandinavian Journal of Public Health. 2008;36:744–752. doi: 10.1177/1403494808090634. [DOI] [PubMed] [Google Scholar]
- Gardner JP, Li S, Srinavasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- O'Donnel CJ, Demissie S, Kimura M, Levy D, Gardner JP, White C, D'Agostino RB, Wolf PA, Polak J, Cupples LA, Aviv A. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1165–1171. doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda M, Ichikawa SC, Kanda T, Sumino H, Kobayashi I. Hormone replacement therapy increases plasma level of angiotensin II in postmenopausal hypertensive women. American Journal of Hypertension. 2001;14:206–211. doi: 10.1016/S0895-7061(00)01253-X. [DOI] [PubMed] [Google Scholar]
- Kejariwal D, Stepien KM, Smith T, Kennedy H, Hughes DA, Sampson MJ. Lack of association of colonic epithelium telomere length and oxidative DNA damage in type 2 diabetes under good metabolic control. BMC Endocrine Disorders. 2008;8:12. doi: 10.1186/1472-6823-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasim H, Al-Daghri N, Chetty R, McTernan PG, Barnett AH, Kumar S. Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in South Asians. Cardiovascular Diabetology. 2006;5:10. doi: 10.1186/1475-2840-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK–eNOS pathway. International Journal of Obesity. 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- Gordon LG, Harten IA, Patti ME, Lichtenstein AH. Reduced adiponectin and HDL-cholesterol without C-reactive protein: clues to the biology of premature atherosclerosis in Hutchinson–Gilford Progeria syndrome. Journal of Pediatrics. 2005;146:336–341. doi: 10.1016/j.jpeds.2004.10.064. [DOI] [PubMed] [Google Scholar]
- Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Hormone and Metabolic Research. 2007;39:9–13. doi: 10.1055/s-2007-956518. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochemical and Biophysical Research Communications. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- Son BK, Akishita M. Vascular calcification and anti-aging. Clinical Calcium. 2008;18:912–917. [PubMed] [Google Scholar]
- Son BK, Akishita M, Iijima K, Kozaki K, Maemura K, Eto M, Ouchi Y. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-alpha on vascular muscle cell calcification: regulation of growth arrest-specific gene 6-mediated survival pathway by adenosine 5′-monophosphate-activated protein kinase. Endocrinology. 2008;149:1646–1653. doi: 10.1210/en.2007-1021. [DOI] [PubMed] [Google Scholar]
- Starr JM, Shiels PG, Harris SE, Pattie A, Pearce MS, Relton CL, Deary IJ. Oxidative stress, telomere length and biomarkers of physical aging in a cohort aged 79 years from the 1932 Scottish Mental Survey. Mechanisms of Ageing and Development. 2008;129:745–751. doi: 10.1016/j.mad.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthorn RM. Accelerated telomere shortening in response to life stress. PNAS. 2004;101:17312–17317. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews. Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic–pituitary–adrenal axis in the pathogenesis of the metabolic syndrome: neuroendocrine and target-tissue-related causes. International Journal of Obesity and Related Metabolic Disorders. 2000;24:S50–S55. doi: 10.1038/sj/ijo/0801278. [DOI] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging. Hormones. 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Cawthorn RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert KE, Misty Y, Hstlings R, Poolman T, Niklason L, Williams B. Angiotensin II mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells vita telomere-dependent and independent pathways. Circulation Research. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlatou MG, Mastorakos G, Lekakis I, Liatis S, Vamvakou G, Zoumakis E, Papssotiriou I, Rabavilas AD, Katsilambros N, Chrousos GP. Chronic administration of an angiotensin II receptor antagonist resets the hypothalamic–pituitary–adrenal (HPA) axis and improves the affect of patients with diabetes mellitus type 2: preliminary results. Stress. 2008;11:62–72. doi: 10.1080/10253890701476621. [DOI] [PubMed] [Google Scholar]
- Diaz VA, Mainous AG, Player MS, Everett CJ. Telomere length and adiposity in a racially diverse sample. International Journal of Obesity. 2010;34:261–265. doi: 10.1038/ijo.2009.198. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, Mohan V. Telomere shortening and metabolic/vascular diseases. Indian Journal of Medical Research. 2007;125:441–450. [PubMed] [Google Scholar]
- Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendrop RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS ONE. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension and leukocyte telomere length in men from the Framingheart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]