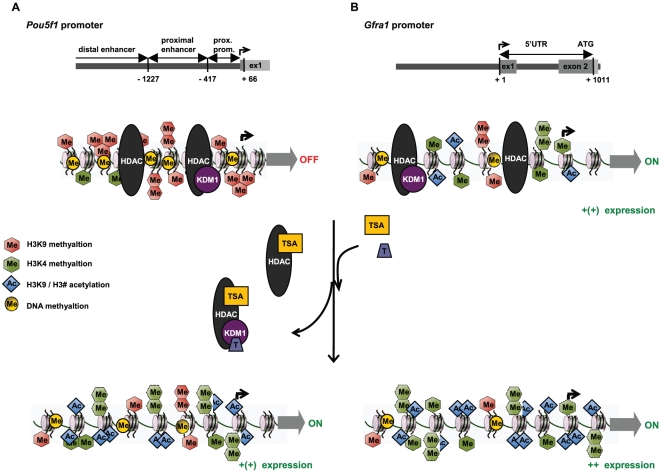
Figure 9. Epigenetic remodeling in Pouf5f1 and Gfra1 promoter regions - a proposed model to explain changes in gene expression in GC-1 cells after Tranylcypromine and TSA treatment.
(A) In untreated GC-1 cells (regular growth conditions), repressive epigenetic marks (e.g. H3K9 methylation and CpG methylation) keep the Pou5f1 promoter silent, by creating a tight chromatin structure and preventing the binding of the transcription machinery. Multi-protein complexes that contain writers of repressive epigenetic marks, e.g. HDACs and/or HDAC/KDM1, create a repressive chromatin state. Blocking these enzymes using TSA (targets HDACs) and/or tranylcypromine (represses KDM1) results in the accumulation of activating epigenetic marks (H3K4 methylation, H3 acetylation), thereby making it accessible for transcription factors and the transcription machinery, which eventually results in the induction of Pou5f1 expression. (B) Repressive and activating epigenetic marks at the promoter are responsible for the weak expression of Gfra1 in untreated GC-1 cells. The exposure of GC-1 cells to tranylcypromine and TSA blocks the gene silencing activities of HDACs and/or HDAC/KDM1 and allows for the accumulation of activating epigenetic marks. Thus the treatment shifts the epigenetic landscape at the Gfra1 promoter to a more active state and eventually triggers a stronger transcription of Gfra1. Histone deacetylase (HDAC), lysine-specific demethylase 1 (KDM1), tranylcypromine (T), trichostatin A (TSA).