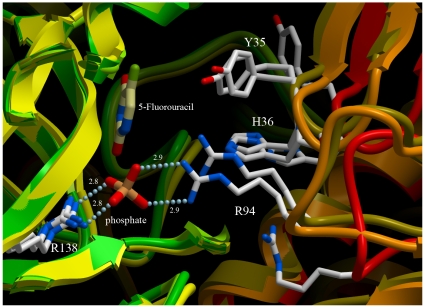
Figure 4. Inter-domain flexibility of hUPP1.
Illustration highlights conformational changes at the dimer interface proximate to the active site, overlaying the 5-FU-bound structure (gold), the BAU-bound structure (orange), and ligand-free structure (red). Despite a lack of molecular contacts between residues from the partnering subunit and the 5-FU ligand, the critical residues for binding natural substrates adopt conformations close to those seen in the BAU-bound structure, where they are stabilized by the formation of favourable molecular interactions, and not the conformations revealed in the ligand-free structure. The location of the phosphate ion from the BAU-bound structure is shown for orientation, but not found to be occupied in the 5-FU-bound structure.