Figure 4. Interaction of L-FABP with carnitine palmitoyl transferase I (CPT1) determined by circular dichroism.
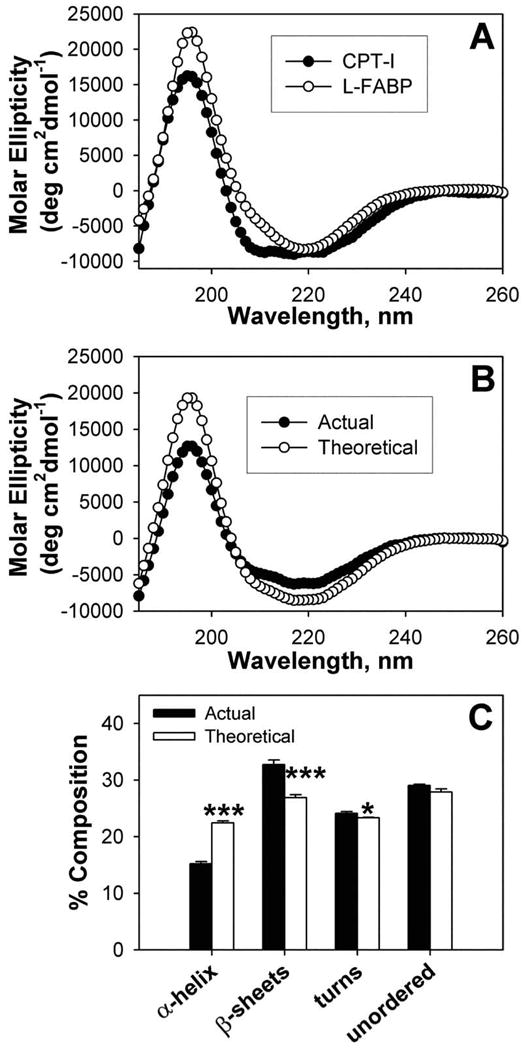
(A) Individual far-UV circular dichroic (CD) spectra of WT CPTI C-terminal 89-residue peptide (filled circles) and an equal amino acid molarity of L-FABP (open circles). (B) Comparison of the far-UV CD spectra of an equal amino acid molarity mixture of WT CPT peptide and L-FABP obtained experimentally (actual, filled circles) and the theoretically expected spectrum (theoretical, open circles) if no conformational change occurred (i.e. the average of the two proteins). (C) Proportion of secondary structures (e.g. α–helix, β-sheet, turn, unordered) in equal molarity mixtures of CPT peptide and L-FABP obtained experimentally (actual, filled bars) and the theoretically expected (theoretical, open bars). Asterisks represent significant differences between the actual and theoretical for each compositional component; * P < 0.05; *** P < 0.001.
