Figure 1. Schematic illustration of Notch structure and pathway activation.
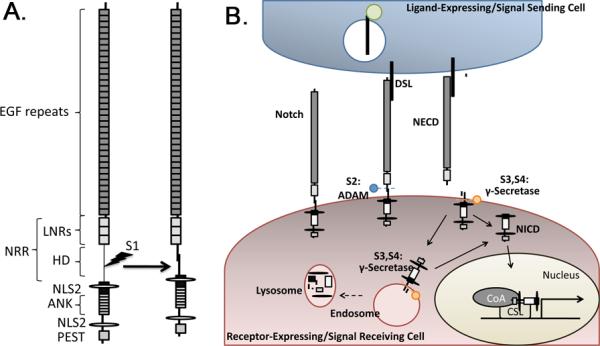
(A) Notch receptors have an extracellular domain composed of reiterated Epidermal Growth Factor (EGF)-like repeats and a conserved negative regulatory region (NRR) consisting of Lin12/Notch repeats (LNRs) and a heterodimerization (HD) domain. The intracellular portion of Notch contains repeated ankyrin (ANK) repeats, nuclear localization signals (NLS) and a PEST domain that controls receptor half life. Vertebrate Notch undergoes S1 cleavage within the secretory pathway to generate the heterodimeric receptor that is found on the cell surface. (B) Notch is activated by binding to ligands of the Delta/Serrate/Lag-2 (DSL) family. The ligands are ubiquitinated (green circle) and internalized into signal sending cells before and/or after receptor activation. Activated Notch undergoes sequential cleavage, initially at the S2 site by members of the ADAM family of metalloproteases (blue ball), and then at the S3 and S4 sites by γ-secretase (orange circle). S2 cleavage occurs at the cell surface and releases the Notch extracellular domain (NECD) from the heterodimer. γ-secretase mediated cleavages take place on the plasma membrane and/or in endosomes. These cleavages release the Notch intracellular domain (NICD), which translocates to the nucleus where it interacts with members of the CBF1/Su(H)/Lag-1 (CSL) family of transcription factors, and recruits co-activators (CoA) to activate transcription of Notch target genes. NICD signaling is terminated by lysosomal degradation.
