Abstract
The parasitic protozoan, Leishmania, survives in harsh environments within its mammalian and sand fly hosts. Secreted proteins likely play critical roles in the parasite’s interactions with its environment. As a preliminary identification of the spectrum of potential excreted/secreted (ES) proteins of Leishmania infantum chagasi (Lic), a causative agent of visceral leishmaniasis, we used standard algorithms to screen the annotated L. infantum genome for genes whose predicted protein products have an N-terminal signal peptide and lack transmembrane domains and membrane anchors. A suite of 181 candidate ES proteins were identified. These included several that were documented in the literature to be released by other Leishmania spp. Six candidate ES proteins were selected for further validation of their expression and release by different parasite stages. We found both amastigote-specific and promastigote-specific released proteins. The ES proteins of Lic are candidates for future studies of parasite virulence determinants and host protective immunity.
Keywords: Leishmania, genome, secretory pathway, excreted/secreted proteins, Trypanosomatid
INTRODUCTION
Leishmania spp. are vector-borne protozoan parasites that cause a group of diseases prevalent primarily in impoverished populations of 88 nations [1], collectively called leishmaniasis. Leishmania are obligate intracellular pathogens residing primarily in mononuclear phagocytes of infected humans and other mammals. Major clinical syndromes include cutaneous, mucosal and visceral leishmaniasis (VL) [1,2]. L. chagasi, which is now thought to be the same species as L. infantum [3,4] (referred to as Lic hereafter), is the most common cause of VL in the New World. VL, the most severe form of leishmaniasis, has an incidence of approximately 500,000 new cases each year, although most cases likely go unreported. VL is characterized by fever, enlarged liver and spleen, anemia and progressive weight loss, and is fatal when left untreated, causing ~57,000 deaths annually. The disease incidence is on the rise due to urbanization and risk of co-infection with HIV [5]. The vast majority of VL is found in Brazil, India, Sudan, Bangladesh and Nepal [6]. Control of these diseases is complicated by difficulty in access to health care, toxicity and expense of treatment regimens, and a lack of a protective vaccine.
Secreted proteins play important roles in the infection process and suppression of host immune systems by both prokaryotic and eukaryotic pathogenic organisms [7,8,9]. Identification of excreted/secreted (ES) proteins of Leishmania could provide insight into mechanisms through which the parasite survives the environmental challenges encountered during its digenetic life cycle. These include transmission between the insect vector and the mammal, entry into host tissue and host macrophages, establishment and maintenance of a parasitophorous vacuole within the infected macrophage, acquisition of nutrients from this intracellular location, and modulation of local and systemic host immune factors. Furthermore, it is known that L. infantum promastigote culture filtrates elicit a strong immune response that is protective against L. major infection in BALB/c mice [10,11], and L. infantum ES antigens produce a long lasting and strong protective effect against canine VL [12]. Thus, some ES proteins could also be a source of vaccine antigens that could provide lasting immune protection.
Despite their importance, the proteins secreted from Leishmania have not been systematically and comprehensively catalogued. Some ES proteins of L. donovani have been identified based on the presence of their enzymatic activity in parasite culture supernatants. These include an acid phosphatase [13], a chitinase [14], a histidine acid phosphatase [15], and a P1/S1 nuclease [16]. Antibodies raised against culture supernatants of L. major parasites were used to screen expression libraries to identify ES proteins. This approach yielded proteases, heat shock proteins, spermidine synthase, ubiquitin ligase, ribosomal and a few unknown proteins [17]. Although valid, the approach of examining proteins in extracellular media of cultured parasites is inevitably plagued by the concern that some proteins result from low level parasite lysis, no matter how healthy the culture. Now that three Leishmania genomes are available, there is the opportunity to systematically search for ES proteins of leishmania in silico and document their expression and release experimentally.
Several of the reported ES proteins bear an N-terminal classical signal peptide indicating that they enter the endoplasmic reticulum (ER)-based secretory pathway. This prompted us to analyze the genome of L. infantum to predict its suite of putatively secreted proteins. In this process, we predicted 181 proteins to be secreted from Lic. We chose six candidate proteins for further analysis and demonstrate that five of these are indeed present in concentrated culture supernatants of Lic. The list included some previously unidentified ES proteins of this parasitic protozoan pathogen. Detailed studies of these and other candidates could shed light on yet undiscovered aspects of Leishmania pathogenesis and illuminate possible strategies for disease prevention.
MATERIALS and METHODS
Bioinformatic Analysis
The complete annotated genome of L. infantum was downloaded from the Sanger Institute (ftp://ftp.sanger.ac.uk/pub/pathogens/L_infantum/DATASETS/). We used the dataset released on 4.26.2007. SignalP (http://www.cbs.dtu.dk/services/SignalP/), TargetP (http://www.cbs.dtu.dk/services/TargetP/), TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0/) and PHOBIUS (http://phobius.cgb.ki.se/) were used to predict the presence of signal peptides, localization in the cell and absence of transmembrane helices respectively. GPI-SOM (http://gpi.unibe.ch/) and Big PI Predictor (http://mendel.imp.ac.at/sat/gpi/gpi_server.html) were used to search for GPI anchor sites. Functional domains in candidate proteins were identified using Pfam HMM (http://pfam.janelia.org/) and Prosite (http://ca.expasy.org/tools/scanprosite/). Searches for homologous proteins were performed by BLAST at NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Parasites and Bacteria
A Brazilian strain of L. chagasi (MHOM/BR/00/1669), originally isolated from a visceral leishmaniasis patient, was used. Parasites were passaged serially through male golden hamsters to maintain virulence. Amastigotes were isolated from infected hamster spleens and were cultivated in Hemoflagellate mOdified Minimal Essential Medium (HOMEM) [18] supplemented with 10% fetal calf serum (FCS), at 26°C, pH 7.4, under which conditions they convert into promastigotes. Promastigotes retain their virulence when maintained in vitro for up to three weeks after isolation [19]. Amastigotes were cultured in a medium containing 20% FCS at 37°C, 5% CO2, pH 5.5, as described previously for Leishmania donovani [20]. We used a line derived from Lic, called LcJ, which cycles in axenic culture between amastigotes at 37°C, pH 5.0 and promastigotes at 26°C, pH 7.4 [21]. Unlike other long-term cultivated Lic lines, LcJ parasites continue to retain their infectivity for mice, and to express virulence associated proteins, as long as they are cycled between the life stages every three weeks [21] All LcJ parasites used in our experiments were three weeks post conversion to ensure complete stage conversion. Metacyclic populations of Lic were isolated according to their buoyant density as described previously [22].
All procedures with animals were approved by the institutional Animal Care and Use Committees at the University of Iowa and the Iowa City VA Medical Center.
E. coli strains were routinely cultured in Luria Bertani broth at 37°C . Antibiotics were used at the following concentrations (µg/ml): ampicillin, 100; kanamycin, 50; carbenicillin, 100; chloramphenicol, 30.
Cloning and recombinant protein expression
Genes of interest were amplified by polymerase chain reaction (PCR) from L. chagasi genomic DNA as per standard protocols [23]. Oligonucleotide primers were purchased from Integrated DNA Technologies (Coralville, IA). Full-length genes including signal peptides were cloned into either pET28a (Novagen, Gibbstown, NJ) or pDEST17 (Invitrogen, Carlsbad, CA) vector, in frame with an N-terminal His6 tag, for expression in E. coli. Clones were verified for sequence accuracy and proper fusion to the His tag. DNA sequencing was done at the University of Iowa DNA core facility with the Automated DNA sequencer, Model 3730 (Applied Biosystems). DNA sequences were analyzed with FinchTV software and BLASTN and BLASTP [24] available through the L. infantum GeneDB (http://www.genedb.org/genedb/linfantum/). Plasmid constructs were introduced into E. coli strains BL21(DE3) (for pET28a) or BL21-A1 (for pDEST17) by transformation. Expression was induced by the addition of either IPTG or arabinose to the cultures, respectively. Proteins were separated by SDS-PAGE and visualized by staining with Coomassie Brilliant Blue per standard protocols [23]. The presence of the N-terminal His tag was confirmed on immunoblots using an anti-Penta His antibody (Molecular Probes, Eugene, OR) and a peroxidase conjugated goat anti mouse secondary antibody (BioRad, Richmond, CA). Immunoblots were developed by an enhanced chemiluminescence kit (Denville Scientific, Metuchen, NJ).
Antibody generation and immunoblots
Antiserum to recombinant parasite proteins was raised in rats as per approved protocols at Cocalico Biologicals (Reamstown, PA). Slices containing the recombinant proteins were excised from polyacrylamide gels and used for immunization. Sera prior to and after immunizations were analyzed against E. coli – expressed recombinant protein for recognition specificity by standard immunoblots [23]. Parasite samples were analyzed using 1:2000 dilution of the primary antibodies and 1:10,000 dilution of a peroxidase-conjugated goat anti rat secondary antibody (Invitrogen, Carlsbad, CA), unless otherwise mentioned.
Parasite lysates and supernatants
For total cell lysates, three week old LcJ promastigotes and amastigotes were harvested by centrifugation, followed by lysis in standard SDS-PAGE sample buffer containing either DTT or βME or both, as the reducing agent. For supernatants, stationary phase LcJ promastigotes, or LcJ amastigotes were suspended for 24 hrs in serum-free BSA-free medium, and then centrifuged (1300 ×g, 10 minutes) to remove parasites. The resulting supernatant was carefully removed and passed through a 0.22 µm filter. Centrifugal concentration cells (Millipore, Bedford, MA) with a molecular weight cutoff of 10 kD were used to concentrate supernatants 50-fold. Samples were reduced by boiling in SDS-PAGE sample buffer prior to electrophoresis. Proteins from 1 × 107 parasites were used in immunoblots for detection of ES protein in cell lysates, whereas concentrated supernatants from 5 × 107 parasites were compared for the presence of a parasite protein in the extracellular medium. Similar numbers of parasites were also used for immunoblots containing log, stationary and metacyclic stage Lic promastigotes. Immunoblots shown are representative of results obtained from 2–3 independent sets of samples.
Visceral Leishmaniasis patient serum
Serum was obtained from patients with symptomatic visceral leishmaniasis in Natal, Brazil. Disease was documented by characteristic symptoms, i.e., parasites viewed in bone marrow smears, positive serology and response to therapy. All subjects or their legal guardian signed an informed consent. The protocol was approved by the Universidade Federal do Rio Grande do Norte Ethical committee (CEP-UFRN 172–06, CONEP 13745, CAAE 0130.0.051.069–06), the NIH, and the University of Iowa IRB.
Immunoblots were performed using 1:5000 dilution of the pooled VL serum in 5% milk and 1:10,000 dilution of a peroxidase-conjugated goat anti-human secondary antibody, as per standard protocols.
Confocal Microscopy
5 × 107 parasites were stained with carboxyfluorosceinsuccinimidyl ester (CFSE) as described previously [25], and allowed to adhere to 12 mm coverslips that were pretreated with poly-L-lysine (60 min). Parasites were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.2% Triton X-100 for 7 min. The coverslips were blocked with 5% Normal Goat Serum (NGS)/PBS for 60 min and incubated with 1:20 primary antibody against parasite ES protein of choice for 60 min. This was followed by 60 min incubation with 1:200 Alexa fluor 568 (red) conjugated goat anti-rat IgG. All incubations were done at room temperature. After rinsing with PBS, coverslips were mounted with Vectashield H-1000 (Vector labs, Burlingame, CA) and examined on a BioRad Radiance 2100MP Multiphoton/Confocal Microscope. Images were captured using the Laser Sharp 2000 software and analyzed using ImageJ software. All microscopic studies were performed at the University of Iowa Central Microscopy Research Facility.
Post-imaging analysis
Over 20 confocal micrographs of promastigotes per condition were analyzed for their fluorescence specificity. Promastigote images were divided in half, and the mean gray values (MGV) of the anterior and posterior sections were calculated. In order to determine whether antisera detection was stronger in the anterior versus the posterior end, the following ratio was calculated: (Red MGV of the anterior section / red MGV + green MGV of the anterior section) : (red MGV of the posterior section / red MGV + gGreen MGV of the posterior section). MGV were calculated using ImageJ software, and statistical analyses were performed on GraphPad Prism 5.
Approvals
Studies using Brazilian human sera were approved by the following Institutional Review Boards: the University of Iowa, the Universidade Federal do Rio Grande do Norte, the NIH and the Brazilian government. The Brazilian IRB is registered with the NIH.
RESULTS AND DISCUSSION
Bioinformatic identification of ES proteins
The genomes of four Leishmania species, i.e., L. infantum (identical to Lic, the focus of this study), L. major, L. braziliensis and L. mexicana have been sequenced and made publicly available [26,27]. We analyzed the annotated genes of the Lic genome for amino-acid sequence characteristics that predict ES proteins and identified a suite of these predicted proteins. The secretory pathway algorithms applied herein contrast to targeting signals reported to direct proteins toward organelles of the kinetoplastids. For instance, targeting to glycosomes in T. brucei requires only a tripeptide in the C-terminus, whereas targeting to glycosomal membranes requires a 15-mer internal binding site for the import protein PEX19 and a transmembrane domain [28,29]. Targeting of proteins for synthesis in the endoplasmic reticulum requires a signal sequence, whereas targeting for retention in the ER was shown to require the C-terminal tetrapeptide MDDL [30].
As a first step, we screened the entire annotated genome using the TargetP [31] algorithm, which predicts the subcellular localization of eukaryotic proteins. This yielded a subset of proteins that were predicted to possess a secretory pathway signal peptide and would transit through the ER. Next, this set was analyzed for the presence of a signal peptide using the SignalP algorithms [32]. We chose only those genes that fulfilled the criteria in both the algorithms that are used by the SignalP program. These candidates were further screened for transmembrane domains using TMHMM and PHOBIUS [33] programs. We included all proteins that were predicted by at least one program to have no transmembrane-like domain other than the signal peptide. The resultant set consisted of 222 candidates from Lic that are predicted to enter the secretory pathway, have signal peptides and lack any transmembrane domains. GPI anchors are commonly found in Leishmania proteins that are localized on the surface of the parasite. Therefore, we also confirmed the absence of GPI anchors in these proteins using the algorithms big-PI Predictor and GPI-SOM [34,35].
This approach yielded 181proteins, 115 of which were hypothetical proteins and 66 of which were known or homologues of known proteins. These 66 proteins included candidates that are involved in processes such as sugar and lipid metabolism and oxidoreduction, as well as housekeeping proteins, degradative enzymes, chaperones and others (Table 1S). Whereas several of the hypothetical proteins also possessed domains suggestive of their possible functions, the majority did not (Table 2S). The approach was validated by the finding of a number of proteins already reported as Leishmania spp. secreted proteins (e.g., acid phosphatase [13], chitinase [14], histidine acid phosphatase [15], P1/S1 nuclease [16], dihydrolipoamide acetyltransferase [17], and protein disulphide isomerase [17].
Based on their annotated identities, we selected six ES candidates that might influence the host environment and/or cause protective immunity for further characterization. Of these, P1/S1 nuclease, dihydrolipoamide acetyltransferase (DHA) and protein disulphide isomerase (PDI) were chosen because they are known to be secreted by other Leishmania species and would serve as “positive controls”, in addition to their possible roles in promoting virulence. Another candidate, the peptidyl-prolyl cis-trans isomerase MIP (macrophage infectivity potentiator), was chosen because of its similarity to other known parasite virulence factors. The remaining two were selected on the basis of their possible relevance to host pathways believed to be involved in the infection process and in host recognition. These candidates are listed in Table 1.
Table 1.
Candidate excreted/secreted proteins of L. chagasi infantum.
| TPb | TMHMM | PHOBIUS | Mass (kDa) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Candidate | IDa | Gene | Loc | SPc | TMd | Topology | TMe | SPf | Topology | Predicted | Observed |
| Cell/Sec g | |||||||||||
| ES1 | LinJ18.V3.0450 | Serine carboxypeptidase | S | Y | 1 | i7-29o | 0 | Y | n5-17c29/30o | 51/48 | 58/50 |
| ES2 | LinJ05.V3.0750 | Flavodoxin domain hypothetical protein | S | Y | 0 | o | 0 | Y | n3-21c26/27o | 90/87 | ~60/70 |
| ES3 | LinJ21.V3.0610 | Dihydrolipoamide acetyltransferase | S | Y | 0 | o | 0 | Y | n5-12c23/24o | 43.5/41 | 50/60 |
| ES4 | LinJ36.V3.7280 | Protein disulphide isomerase | S | Y | 1 | i5-24o | 0 | Y | n5-16c20/21o | 53/50 | ~90/60 |
| ES5 | LinJ30.V3.1520 | P1/S1 nuclease | S | Y | 0 | o | 0 | Y | n9-19c24/25o | 35/32 | 30/45 |
| ES6 | LinJ19.V3.0920 | Mip PPIase | S | Y | 1 | o10-32i | 0 | Y | n10- 24c28/29o |
22/19 | 22,18/na |
Gene ID from L. infantum GeneDB.
Prediction of cellular location of protein by TargetP program; “S” indicating entry into secretory pathway.
Presence of signal peptide by SignalP program.
Prediction of transmembrane domain (TM) by TMHMM program. TMHMM considers the signal peptide as a TM.
Prediction of TM by PHOBIUS program.
Prediction of signal peptide by PHOBIUS program.
Predicted molecular mass in the cell or secreted into the medium
Expression/Secretion analysis of candidate ES proteins in LcJ parasites
Lic is a dimorphic organism with a flagellated, extracellular promastigote form and an aflagellate, obligate intracellular amastigote form. Whereas the promastigote develops through several intermediates to the infectious metacyclic form within the gut of the sand fly, the amastigote is the only form within infected mammals. We hypothesized that ES proteins are likely to be expressed by each of these life stages, but the expressed proteins may not necessarily be the same in both life stages. Since there are currently no algorithms to predict the probability of expression in different life stages, we investigated the possible presence of ES proteins in cell lysates and culture supernatants of both promastigotes and amastigotes of Lic. Towards this purpose, we used the stage-converting LcJ line that was derived from Lic. Axenic LcJ amastigotes have been previously demonstrated to be similar to true amastigotes based on their expression of the amastigote-specific A2mRNA [36], the absence of the promastigote-specific ARL3A transcript [37], the absence of lipophosphoglycan (LPG) in amastigote lysates [21], and the release of MSP at similar levels as wild type amastigotes [21].
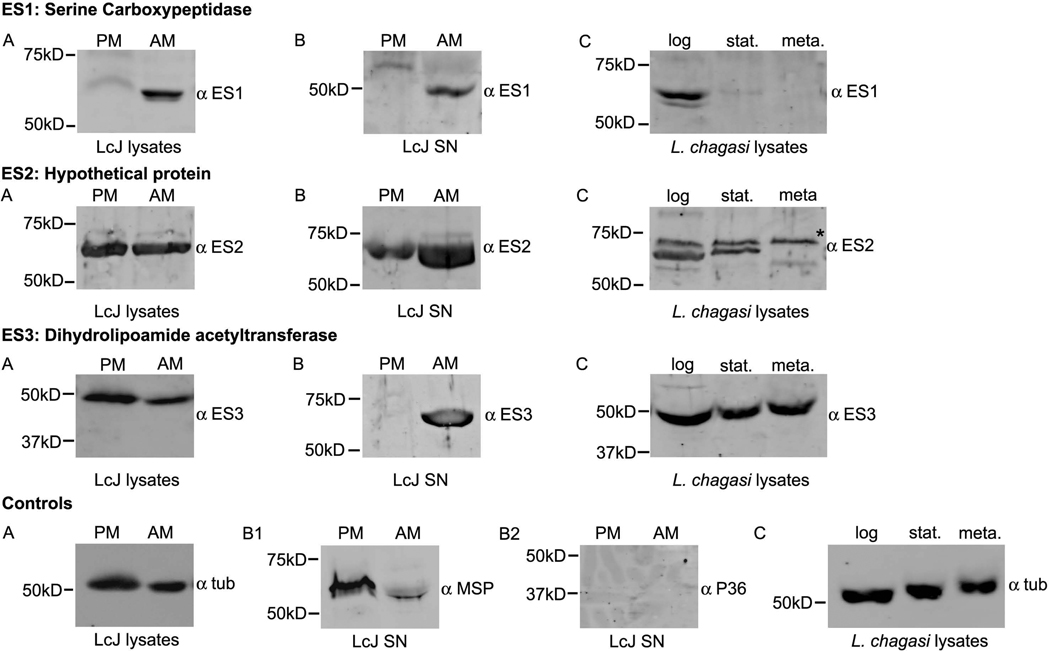
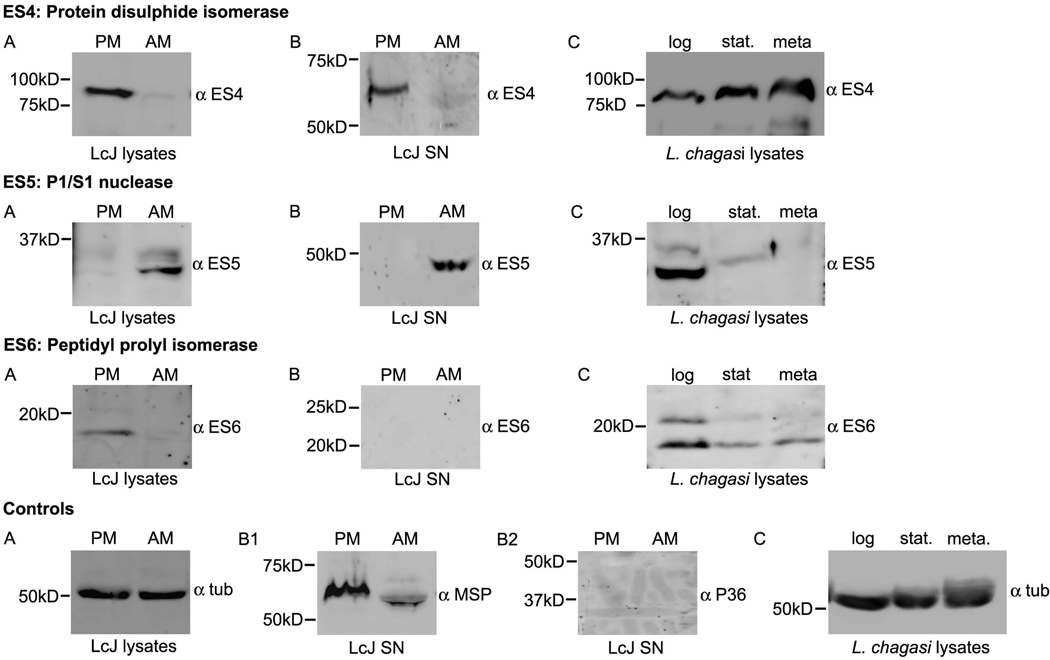
Immunoblots revealed a wide variation in the stage-specific expression and secretion patterns of the candidate ES proteins (Fig. 1 and Fig. 2). There were several differences between the predicted and observed sizes of some protein candidates (Table 1). These differences in size could be due to modifications occurring in the parasite, since all the recombinant E. coli proteins all displayed their expected sizes (Table 3S). Identical immunoblots of cell lysates were probed with α-tubulin as a loading control. Immunoblots of concentrated culture supernatants were probed with polyclonal antisera for P36 and for MSP (also called GP63). P36 is an internal protein of Lic and thereby served as a negative control to rule out cell lysis [19]. Major surface protein (MSP), a protein known to be released from Lic, served as a positive control known secreted protein [21]. As expected, only MSP (Fig. 1 and Fig. 2) but not P36 was observed in the supernatants of all samples.
Fig. 1.
Expression and secretion analysis of candidate proteins ES1, 2, and 3. (A) Total protein extracted from 1 × 107 LcJ promastigotes (PM) or amastigotes (AM), or (B) concentrated supernatants derived from 5 × 107 PM or AM were separated by SDS-PAGE and probed with 1:2000 primary ES protein-specific antisera. After treatment with secondary antibody, immunoblots were visualized by enhanced chemiluminescence. (C) Total lysates of log, stationary or metacyclic stage Lic promastigotes were similarly analyzed with immunoblots. Identical blots were also probed with antibody to α-tubulin as a control for equal loading of cell lysates. Immunoblots of supernatant samples were probed for the presence of MSP and absence of P36 to ensure that no significant cell lysis occurred during sample preparation. “*” marks a non-specific band on the ES2 immunoblot that is also present in blots probed with pre-immune serum.
Fig. 2.
Expression and secretion analysis of candidate proteins ES 4, 5, and 6. (A) Total protein extracted from 1 × 107 LcJ promastigotes (PM) or amastigotes (AM), or (B) concentrated supernatants derived from 5 × 107 PM or AM were separated by SDS-PAGE and probed with 1:2000 primary ES protein-specific antisera. After treatment with secondary antibody, immunoblots were visualized by enhanced chemiluminescence. (C) Total lysates derived from log, stationary or metacyclic stage Lic parasites were similarly analyzed with immunoblots. Immunoblots of supernatant samples were probed for the presence of MSP and absence of P36 to ensure that no significant cell lysis occurred during sample preparation.
These expression patterns could provide clues to the roles of these proteins in different stages of the parasite’s life cycle. ES1, which encodes a serine carboxypeptidase/peptidase, was expressed and secreted predominantly by the amastigote form of LcJ (Fig. 1). Closely related serine proteases have been identified as secreted virulence factors in L. amazonensis [38], Trypanosoma cruzi [39], Plasmodium spp. [40], Toxoplasma gondii [41] and parasitic helminths [42,43], that can digest host membrane proteins, collagen, fibrinogen, gelatin and other proteins and thereby facilitate host tissue invasion and parasite dissemination [44]. Unlike the L. amazonensis serine protease expressed by promastigotes, our ES1 candidate was expressed and secreted exclusively by the LcJ amastigote stage. ES2 is a hypothetical protein that contains a flavodoxin domain, and was expressed equally by both LcJ promastigotes and amastigotes. Whereas both forms secreted the protein, amastigotes secreted significantly greater quantities than promastigotes (Fig. 1). Since Leishmania are susceptible to killing by reactive oxidant species generated during phagocytosis by macrophages [45], it is possible that the secreted ES2 is part of the protection mechanism adopted by promastigotes to reduce oxidant-mediated killing.
Lic ES3 is annotated as a dihydrolipoamide acetyltransferase with attachment sites for both lipoic acid and biotin. Also known as E2, this protein is an integral part of the pyruvate dehydrogenase complex in mitochondria. Its homolog in L. major has been previously reported to be secreted [17]. Our immunoblots suggested that ES3 is expressed equally by both life forms, but is secreted only by amastigotes (Fig. 1).
Our next candidate, ES4, is a protein disulphide isomerase that was previously reported to be a virulence factor [46] secreted by L. major [17]. We observed that LcJ ES4 was expressed and secreted only by the promastigote form (Fig. 2). Most PDIs are ER residents that are involved in protein folding [47], but Lic ES4 and its L. major homolog LmPDI are released despite having an ER retention-like signal (EEDL) [17]. LmPDI activity has been shown to serve an essential function in promastigotes [46], and Lic ES4 expression progressively increased as promastigotes developed from less virulent (log phase) to more virulent (metacyclic) forms. These observations could signify a possible role of Lci ES4 in metacyclic promastigotes.
We also analyzed a homolog of a Class I secretory nuclease that was previously reported as a secreted nuclease of L. donovani [16]. Lic ES5 encodes a P1/S1 nuclease that can hydrolyze all nucleic acids. Unlike its counterpart in L. donovani, which is expressed in both life stages, Lic ES5 was expressed and secreted only by LcJ amastigotes (Fig. 2). Since the Leishmania spp. parasites are purine auxotrophs, secreted nucleases may hydrolyze host-derived nucleic acids and support growth of the parasite in a nutritionally-restricted environment [48]. A change in Mr on SDS-PAGE could additionally suggest that secreted Lic ES5 is post-translationally modified.
Our final candidate, Lic ES6, is a homolog of a peptidyl prolyl cis-trans isomerase initially identified in Legionella pneumophila and named macrophage infectivity potentiator (Mip) for its role in macrophage infection [49]. Mip is a secreted protein reported to contribute to the virulence of the parasitic protozoan Trypanosoma cruzi [50], and is expressed by several other bacterial pathogens [51,52]. Our immunoblots showed that ES6 is expressed only by LcJ promastigotes but not amastigotes, and is not present in concentrated culture supernatants of either LcJ promastigotes or amastigotes suggesting that it was not secreted by Leishmania species (Fig. 2). Consistent with these observations, Mip homologs in several bacterial pathogens are surface-localized [53,54] despite the presence of a signal peptide. Nonetheless, it supports the assumption that in silico prediction of secretion requires experimental validation of the same.
Differences between the predicted sizes of proteins and observed sizes on immunoblots suggested post-translational processing (Figs. 1 and 2). Comparing secreted versus parasite cell-associated ES1 and ES4, and metacyclic versus logarithmic cell-bound ES6, revealed the proteins migrated further into gels indicating processing (e.g., proteolytic cleavage). In contrast, secreted ES3 and ES5 migrated more slowly than cell-bound forms, suggesting different processing events had occurred (e.g., glycosylation). Both the cell-bound and the extracellular ES2 protein seemed to have undergone processing, as the observed size (~65kD) was smaller than its predicted size (90kD). Similarly, cell bound ES4 protein was likely modified, since its observed relative migration (~90kD) was higher than the predicted size of 53kD.
Expression in metacyclics
Metacyclogenesis is a developmental process that occurs within the gut of the sand fly, the insect vector of Leishmania spp., during which the less infective procyclic promastigotes transform into highly infective metacyclic promastigotes that are ready for inoculation into a host [55]. Metacyclogenesis is accompanied by changes in morphology and increased expression of several leishmania virulence factors (e.g., MSP and LPG) [56,57]. Metacyclic Lic promastigotes can be isolated from day 7–9 cultures using a ficoll step gradient method [22]. Since metacyclic organisms are poised for infecting the mammalian host, we hypothesized that some ES antigens that are expressed by amastigotes might also be expressed by metacyclics.
Analysis of our selected ES proteins revealed that three of the six candidates are expressed in metacyclic Lic parasites. These were DHA (ES3), the protein disulphide isomerase (ES4) and the non-secreted protein Mip (ES6). Of these, ES3 was the only one that was also expressed in amastigotes. Although ES3 was equally expressed in all the Lic stages (LcJ promastigotes, LcJ amastigotes, Lic logarithmic and stationary promastigotes), it was secreted extracellularly only by amastigotes (Fig. 1). ES4 and ES6 were expressed by all promastigotes (log, stationary, metacyclic) but not by amastigotes. The progressive increased expression of ES4 in log, stationary and metacyclic Lic promastigotes suggested the protein was associated with promastigotes virulence. ES6, our only candidate protein that was not secreted, exhibited two reactive bands in logarithmic promastigotes that corresponded to the expected protein sizes before and after the cleavage of the signal peptide. ES6 expression was reduced in the stationary and metacyclic stages of Lic, and in these stages the presumed mature ES6 [lower relative mass (Mr)] protein dominated (Fig. 2).
Amongst the remaining candidates, ES1 and ES5 were expressed and secreted only by the LcJ amastigotes but not promastigotes. Both these candidates were also expressed in logarithmic phase but not stationary or metacyclic wild type Lic promastigotes (Figs. 1 and 2). The final candidate, ES2, was expressed and secreted by both promastigotes and amastigotes according to LcJ blots, whereas blots of wild type Lic revealed that ES2 was not expressed by metacyclic promastigotes. The highest Mr band in the ES2 immunoblot was also recognized by pre-immune serum blots, and thus was thought to be non-specific (Fig. 1). Overall there was a rough correlation between ES protein expression in the amastigote stage and logarithmic-growth promastigotes. Not surprisingly, immunoblots suggest that stationary parasite preparations were a mixture of logarithmic-type and metacyclic promastigotes.
Immunolocalization in parasites
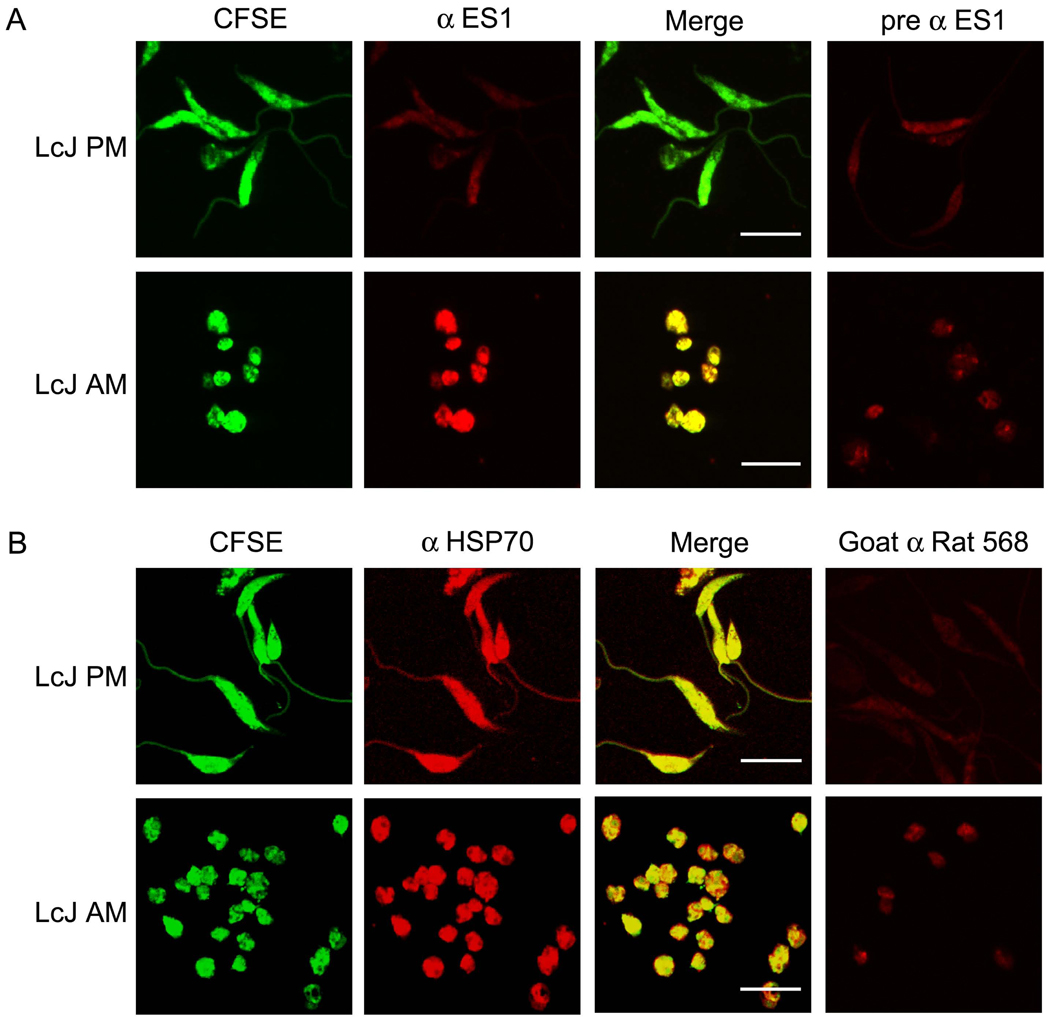
We used confocal microscopy to localize two candidate proteins (ES1 and ES3) with different expression patterns in amastigotes versus promastigotes. LcJ promastigotes or amastigotes were labeled with CFSE, fixed on cover slips and probed with pre-immune or post-immune sera. Control confocal studies with pre-immune sera showed only background staining was present. The Alexafluor-conjugated secondary antibody showed only background staining.
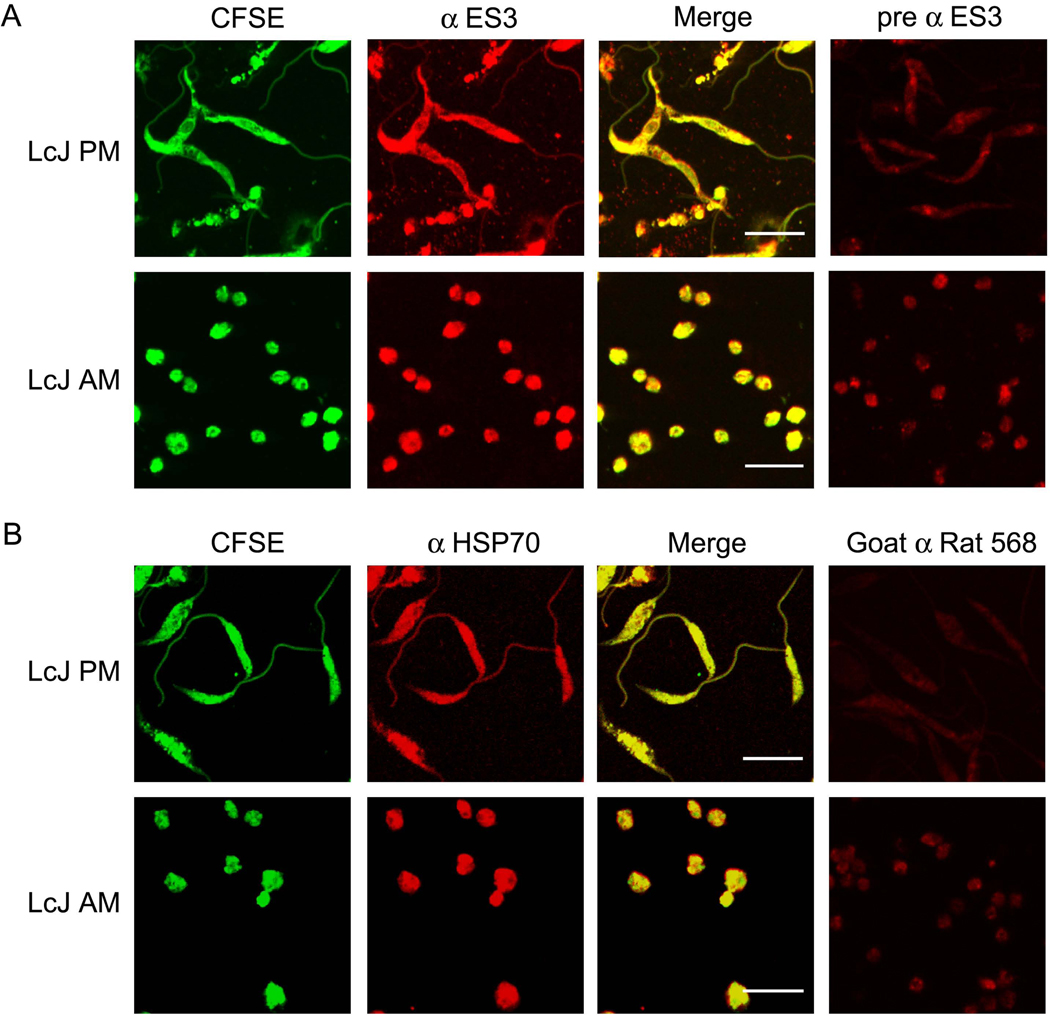
Confocal micrographs (Fig. 3 and Fig. 4) mirrored the data obtained with immunoblots. ES1 immunostaining above background staining with pre-immune serum was observed only in LcJ amastigotes but not promastigotes, whereas ES3 staining above background was seen in both life forms. ES3 appeared to localize to an intense focus of fluorescence near the flagellar pocket of LcJ promastigotes, consistent with its secretion. However, post-imaging comparison of the mean gray values of the pixels in the anterior versus the posterior end of the promastigote revealed that there was no significant focal accumulation of the protein at either end.
Fig. 3.
Immunohistochemical localization of ES1. (A) 5 × 106 CFSE-stained LcJ promastigotes or amastigotes were fixed on 12mm coverslips and permeabilized. Coverslips were probed with primary antiserum to ES1 (α-ES1) followed by Alexafluor 568-conjugated secondary antibody, and visualized by confocal microscopy. (B) Control studies included parasites probed with α-HSP70, which is expressed by both forms of the parasite. No signals were observed in parasite samples probed with α-ES1 pre-immune serum (pre α ES1) or with secondary antibody only (Goat α Rat 568). Scale bar represents 10 microns in all panels.
Fig. 4.
Confocal micrographs of ES3 expression in LcJ parasites. (A) 5 × 106 LcJ promastigotes or amastigotes were stained with CFSE, fixed on 12mm coverslips and permeabilized. Samples were probed with antiserum to ES3 (α-ES3) followed by Alexafluor 568-conjugated secondary antibody, and visualized by confocal microscopy as in Figure 3. (B) Control coverslips were incubated with α-HSP70. No colocalization was observed in samples probed with either α-ES3 pre-immune serum (pre α ES3) or with Alexafluor 568-conjugated secondary antibody only (Goat α Rat 568). Scale bar represents 10 microns in all panels.
The pattern of ES3 staining was reminiscent of that of ER staining, with obvious clearance of the nucleus. Antiserum to the Lic heat shock protein 70 (HSP70), a constitutively expressed cytoplasmic protein, was used as the positive control for this study. In a pattern that was distinctly different from that of ES3, HSP70 showed a strong and uniform staining of the entire parasite cytoplasm. Some LcJ amastigotes showed punctuate staining with both ES1 and ES3. However, due to the small size of LcJ amastigotes, it is difficult to attribute the staining to specific sub-cellular compartments.
Recognition by pooled VL serum
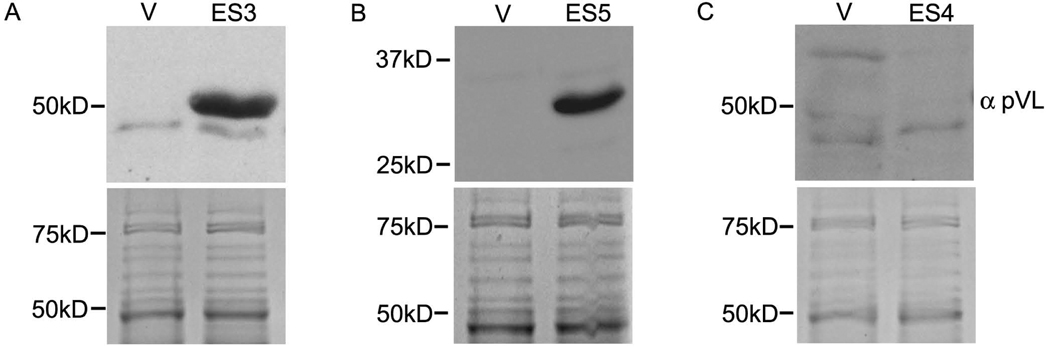
We analyzed the ability of serum from VL patients to recognize recombinant ES antigens synthesized in E. coli. Of the six ES proteins analyzed, we demonstrate that at least two candidates, ES3 and ES5 were recognized by VL patient serum (Fig. 5). Although this does not indicate that the proteins are secreted in vivo during human infection, they do indicate that the proteins are exposed to the human immune system. ES4, which was not recognized by patient serum, served as a negative control. Importantly, both ES3 and ES5 are released extracellularly by the amastigote stage (Fig. 1 and Fig. 2), the parasite form residing intracellularly in an infected mammalian host, leading us to speculate that the ES proteins that are secreted exclusively by the promastigotes (e.g., ES4) might be released only within the sand fly but not the human host.
Fig. 5.
Recognition of ES proteins by pooled VL serum. Total protein obtained from E. coli strains expressing recombinant (A) ES3, (B) ES5 and (C) ES4 protein were subject to immunoblotting with pooled VL patient serum. Protein derived from E. coli strains harboring the empty expression vector (V) but treated to similar induction as the recombinant strain was used as control. Both recombinant ES3 and ES5 were strongly recognized by VL serum, suggesting their possible secretion during in vivo infection. ES4 is representative of the ES proteins that were not recognized. The lower panels show representative coomassie brilliant blue stain of the gels.
This study describes an initial attempt to define the secretome of L. chagasi infantum according to the sequence characteristics of proteins in the genome. We were able to verify experimentally that indeed five of our six candidate proteins are secreted by one or several forms of the pathogen. Furthermore, several proteins predicted by these algorithms to be secrerted were already documented in the literature to be secreted/excreted, further validating the approach.
Despite the above validations, published analyses of the promastigote or “secretome” additionally include a variety of proteins that lack secretory sequence features, suggesting these protozoa possess additional pathways for protein release. Amongst eukaryotic cells, the Leishmania spp. and other trypanosomatid protozoa are unique in their possession of a dense subpellicular network of microtubules, preventing conventional pinocytosis or endocytosis as occurs in other eukaryotic cells. Instead these protozoa have a flagellar pocket, a deep invagination near the anterior site of emerging flagellum, which is the sole site of cellular endocytosis and exocytosis. Considering that the flagellar pocket constitutes less than 5% of the surface area of the cell, protein trafficking through this site occurs at a very high rate. Furthermore, clathrin coated pits and vesicles originating at the flagellar pocket membrane are essential for turnover of the variant surface protein (VSG) in Trypanosoma brucei, including both endocytosis and exocytosis [58,59]. Sorting of membrane-bound proteins within endocytic and exocytic vesicles may involve selective inclusion or exclusion of membrane lipids or anchors including cholesterol, sphingomyelin and GPI anchored proteins [58,59]. In addition to VSG, other GPI anchored membrane proteins have clearly been found released to the external environment [e.g., GP63/MSP of promastigotes [19,60]]. Some proteins exported to the cell surface lack a predicted signal sequence [e.g. gene B protein of L. major; [61]]. The spatial relation between clathrin vesicle-mediated transport and proteins predicted to exit the cell via the secretory pathway is not known. These unpredicted pathways of protein trafficking indicate that there is much to be learned about protein trafficking in the Trypanosomatid protozoa.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Jian Shao for help with confocal microscopy. This work was performed during the tenure of SD on NIH Training grant T32 HL 0712, and during the support of AK by the Iowa Center for Research by Undergraduates. The work was funded in part by the following research grants: R01 AI059451 from the National Institutes of Health (JED and MEW), NIH grants AI045540, AI067874, AI076233 and AI048822 (MEW), NIH grant AI-30639 (SJ and MW) and a Merit Review grant (MEW) from the Department of Veterans’ Affairs.
Abbreviations
- ES
excreted/secreted
- Lic
Leishmania infantum chagasi
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- 1.Pearson RD, Sousa AQ. Clinical spectrum of Leishmaniasis. Clin Infect Dis. 1996;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 3.Mauricio IL, Stothard JR, Miles MA. The strange case of Leishmania chagasi. Parasitol Today. 2000;16:188–189. doi: 10.1016/s0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- 4.Momen H, Pacheco RS, Cupolillo E, Grimaldi Junior G. Molecular evidence for the importation of Old World Leishmania into the Americas. Biol Res. 1993;26:249–255. [PubMed] [Google Scholar]
- 5.Sinha PK, Pandey K, Bhattacharya SK. Diagnosis & management of leishmania/HIV co-infection. Indian J Med Res. 2005;121:407–414. [PubMed] [Google Scholar]
- 6.Sinha PK, Ranjan A, Singh VP, Das VN, Pandey K, Kumar N, Verma N, Lal CS, Sur D, Manna B, Bhattacharya SK. Visceral leishmaniasis (kala-azar)--the Bihar (India) perspective. J Infect. 2006;53:60–64. doi: 10.1016/j.jinf.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Anantharaman V, Iyer LM, Balaji S, Aravind L. Adhesion molecules and other secreted host-interaction determinants in Apicomplexa: insights from comparative genomics. Int Rev Cytol. 2007;262:1–74. doi: 10.1016/S0074-7696(07)62001-4. [DOI] [PubMed] [Google Scholar]
- 8.Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol. 2007;10:582–587. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stavrinides J, McCann HC, Guttman DS. Host-pathogen interplay and the evolution of bacterial effectors. Cell Microbiol. 2008;10:285–292. doi: 10.1111/j.1462-5822.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosa R, Marques C, Rodrigues OR, Santos-Gomes GM. Immunization with Leishmania infantum released proteins confers partial protection against parasite infection with a predominant Th1 specific immune response. Vaccine. 2007;25:4525–4532. doi: 10.1016/j.vaccine.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Rosa R, Rodrigues OR, Marques C, Santos-Gomes GM. Leishmania infantum: soluble proteins released by the parasite exert differential effects on host immune response. Exp Parasitol. 2005;109:106–114. doi: 10.1016/j.exppara.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Lemesre JL, Holzmuller P, Cavaleyra M, Goncalves RB, Hottin G, Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23:2825–2840. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 13.Shakarian AM, Dwyer DM. Structurally conserved soluble acid phosphatases are synthesized and released by Leishmania major promastigotes. Exp Parasitol. 2000;95:79–84. doi: 10.1006/expr.2000.4511. [DOI] [PubMed] [Google Scholar]
- 14.Shakarian AM, Dwyer DM. Pathogenic leishmania secrete antigenically related chitinases which are encoded by a highly conserved gene locus. Exp Parasitol. 2000;94:238–242. doi: 10.1006/expr.2000.4493. [DOI] [PubMed] [Google Scholar]
- 15.Joshi MB, Mallinson DJ, Dwyer DM. The human pathogen Leishmania donovani secretes a histidine acid phosphatase activity that is resistant to proteolytic degradation. J Eukaryot Microbiol. 2004;51:108–112. doi: 10.1111/j.1550-7408.2004.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 16.Joshi MB, Dwyer DM. Molecular and functional analyses of a novel class I secretory nuclease from the human pathogen, Leishmania donovani. J Biol Chem. 2007;282:10079–10095. doi: 10.1074/jbc.M610770200. [DOI] [PubMed] [Google Scholar]
- 17.Chenik M, Lakhal S, Ben Khalef N, Zribi L, Louzir H, Dellagi K. Approaches for the identification of potential excreted/secreted proteins of Leishmania major parasites. Parasitology. 2006;132:493–509. doi: 10.1017/S0031182005009546. [DOI] [PubMed] [Google Scholar]
- 18.Berens RL, Brun R, Krassner SM. A simple monophasic medium for axenic culture of hemoflagellates. J Parasitol. 1976;62:360–365. [PubMed] [Google Scholar]
- 19.Yao C, Leidal KG, Brittingham A, Tarr DE, Donelson JE, Wilson ME. Biosynthesis of the major surface protease GP63 of Leishmania chagasi. Mol Biochem Parasitol. 2002;121:119–128. doi: 10.1016/s0166-6851(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 20.Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, Turco SJ, Beverley SM. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao CH, Yao C, Storlie P, Donelson JE, Wilson ME. The major surface protease (MSP or GP63) in the intracellular amastigote stage of Leishmania chagasi. Mol Biochem Parasitol. 2008;157:148–159. doi: 10.1016/j.molbiopara.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao C, Chen Y, Sudan B, Donelson JE, Wilson ME. Leishmania chagasi: Homogenous metacyclic promastigotes isolated by buoyant density are highly virulent in a mouse model. Exp Parasitol. 2008;118:129–133. doi: 10.1016/j.exppara.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T, editors. Molecular cloning: a Laboratory Manual. Plainview, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang HK, Thalhofer C, Duerkop BA, Mehling JS, Verma S, Gollob KJ, Almeida R, Wilson ME. Oxidant generation by single infected monocytes after short-term fluorescence labeling of a protozoan parasite. Infect Immun. 2007;75:1017–1024. doi: 10.1128/IAI.00914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, Peters N, Adlem E, Tivey A, Aslett M, Kerhornou A, Ivens A, Fraser A, Rajandream MA, Carver T, Norbertczak H, Chillingworth T, Hance Z, Jagels K, Moule S, Ormond D, Rutter S, Squares R, Whitehead S, Rabbinowitsch E, Arrowsmith C, White B, Thurston S, Bringaud F, Baldauf SL, Faulconbridge A, Jeffares D, Depledge DP, Oyola SO, Hilley JD, Brito LO, Tosi LR, Barrell B, Cruz AK, Mottram JC, Smith DF, Berriman M. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RM, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch AC, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Muller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O'Neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schafer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer JM, Peterson G, Keller GA, Parsons M, Wang CC. The C-terminal tripeptide of glycosomal phosphoglycerate kinase is both necessary and sufficient for import into the glycosomes of Trypanosoma brucei. FEBS Lett. 1993;316:53–58. doi: 10.1016/0014-5793(93)81735-i. [DOI] [PubMed] [Google Scholar]
- 29.Saveria T, Halbach A, Erdmann R, Volkmer-Engert R, Landgraf C, Rottensteiner H, Parsons M. Conservation of PEX19-binding motifs required for protein targeting to mammalian peroxisomal and trypanosome glycosomal membranes. Eukaryot Cell. 2007;6:1439–1449. doi: 10.1128/EC.00084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- 31.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 32.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Kall L, Krogh A, Sonnhammer EL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Eisenhaber B, Bork P, Eisenhaber F. Prediction of potential GPI-modification sites in proprotein sequences. J Mol Biol. 1999;292:741–758. doi: 10.1006/jmbi.1999.3069. [DOI] [PubMed] [Google Scholar]
- 35.Fankhauser N, Maser P. Identification of GPI anchor attachment signals by a Kohonen self-organizing map. Bioinformatics. 2005;21:1846–1852. doi: 10.1093/bioinformatics/bti299. [DOI] [PubMed] [Google Scholar]
- 36.Charest H, Zhang WW, Matlashewski G. The developmental expression of Leishmania donovani A2 amastigote-specific genes is post-transcriptionally mediated and involves elements located in the 3'-untranslated region. J Biol Chem. 1996;271:17081–17090. doi: 10.1074/jbc.271.29.17081. [DOI] [PubMed] [Google Scholar]
- 37.Cuvillier A, Redon F, Antoine JC, Chardin P, DeVos T, Merlin G. LdARL-3A, a Leishmania promastigote-specific ADP-ribosylation factor-like protein, is essential for flagellum integrity. J Cell Sci. 2000;113(Pt 11):2065–2074. doi: 10.1242/jcs.113.11.2065. [DOI] [PubMed] [Google Scholar]
- 38.Silva-Lopez RE, Coelho MG, De Simone SG. Characterization of an extracellular serine protease of Leishmania (Leishmania) amazonensis. Parasitology. 2005;131:85–96. doi: 10.1017/s0031182004006675. [DOI] [PubMed] [Google Scholar]
- 39.Santana JM, Grellier P, Schrevel J, Teixeira AR. A Trypanosoma cruzi-secreted 80 kDa proteinase with specificity for human collagen types I and IV. Biochem J. 1997;325(Pt 1):129–137. doi: 10.1042/bj3250129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roggwiller E, Betoulle ME, Blisnick T, Braun Breton C. A role for erythrocyte band 3 degradation by the parasite gp76 serine protease in the formation of the parasitophorous vacuole during invasion of erythrocytes by Plasmodium falciparum. Mol Biochem Parasitol. 1996;82:13–24. doi: 10.1016/0166-6851(96)02714-4. [DOI] [PubMed] [Google Scholar]
- 41.Conseil V, Soete M, Dubremetz JF. Serine protease inhibitors block invasion of host cells by Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:1358–1361. doi: 10.1128/aac.43.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salter JP, Lim KC, Hansell E, Hsieh I, McKerrow JH. Schistosome invasion of human skin and degradation of dermal elastin are mediated by a single serine protease. J Biol Chem. 2000;275:38667–38673. doi: 10.1074/jbc.M006997200. [DOI] [PubMed] [Google Scholar]
- 43.Moczon T. A serine proteinase in the penetration glands of the cercariae of Plagiorchis elegans (Trematoda Plagiorchiidae) Parasitol Res. 1996;82:72–76. doi: 10.1007/s004360050071. [DOI] [PubMed] [Google Scholar]
- 44.Silva-Lopez RE, Morgado-Diaz JA, Chavez MA, Giovanni-De-Simone S. Effects of serine protease inhibitors on viability and morphology of Leishmania (Leishmania) amazonensis promastigotes. Parasitol Res. 2007;101:1627–1635. doi: 10.1007/s00436-007-0706-5. [DOI] [PubMed] [Google Scholar]
- 45.Miller MA, McGowan SE, Gantt KR, Champion M, Novick SL, Andersen KA, Bacchi CJ, Yarlett N, Britigan BE, Wilson ME. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J Biol Chem. 2000;275:33883–33889. doi: 10.1074/jbc.M003671200. [DOI] [PubMed] [Google Scholar]
- 46.Ben Achour Y, Chenik M, Louzir H, Dellagi K. Identification of a disulfide isomerase protein of Leishmania major as a putative virulence factor. Infect Immun. 2002;70:3576–3585. doi: 10.1128/IAI.70.7.3576-3585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedman RB, Hirst TR, Tuite MF. Protein disulphide isomerase: building bridges in protein folding. Trends Biochem Sci. 1994;19:331–336. doi: 10.1016/0968-0004(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 48.Hassan HF, Coombs GH. A comparative study of the purine- and pyrimidine-metabolising enzymes of a range of trypanosomatids. Comp Biochem Physiol B. 1986;84:219–223. [PubMed] [Google Scholar]
- 49.Cianciotto NP, Fields BS. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci U S A. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell A, Monaghan P, Page AP. Peptidyl-prolyl cis-trans isomerases (immunophilins) and their roles in parasite biochemistry, host-parasite interaction and antiparasitic drug action. Int J Parasitol. 2006;36:261–276. doi: 10.1016/j.ijpara.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Horne SM, Kottom TJ, Nolan LK, Young KD. Decreased intracellular survival of an fkpA mutant of Salmonella typhimurium Copenhagen. Infect Immun. 1997;65:806–810. doi: 10.1128/iai.65.2.806-810.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leuzzi R, Serino L, Scarselli M, Savino S, Fontana MR, Monaci E, Taddei A, Fischer G, Rappuoli R, Pizza M. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol Microbiol. 2005;58:669–681. doi: 10.1111/j.1365-2958.2005.04859.x. [DOI] [PubMed] [Google Scholar]
- 53.Cianciotto NP, Eisenstein BI, Mody CH, Toews GB, Engleberg NC. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun. 1989;57:1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neff L, Daher S, Muzzin P, Spenato U, Gulacar F, Gabay C, Bas S. Molecular characterization and subcellular localization of macrophage infectivity potentiator, a Chlamydia trachomatis lipoprotein. J Bacteriol. 2007;189:4739–4748. doi: 10.1128/JB.01889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sacks DL. Metacyclogenesis in Leishmania promastigotes. Exp Parasitol. 1989;69:100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- 56.Roberts SC, Wilson ME, Donelson JE. Developmentally regulated expression of a novel 59-kDa product of the major surface protease (msp or gp63) gene family of Leishmania chagasi. J Biol Chem. 1995;270:8884–8892. doi: 10.1074/jbc.270.15.8884. [DOI] [PubMed] [Google Scholar]
- 57.Sacks DL, da Silva RP. The generation of infective stage Leishmania major promastigotes is associated with the cell-surface expression and release of a developmentally regulated glycolipid. J Immunol. 1987;139:3099–3106. [PubMed] [Google Scholar]
- 58.Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol. 2004;53:735–744. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]
- 59.Hung CH, Qiao X, Lee PT, Lee MG. Clathrin-dependent targeting of receptors to the flagellar pocket of procyclic-form Trypanosoma brucei. Eukaryot Cell. 2004;3:1004–1014. doi: 10.1128/EC.3.4.1004-1014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGwire BS, O'Connell WA, Chang KP, Engman DM. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J Biol Chem. 2002;277:8802–8809. doi: 10.1074/jbc.M109072200. [DOI] [PubMed] [Google Scholar]
- 61.Flinn HM, Rangarajan D, Smith DF. Expression of a hydrophilic surface protein in infective stages of Leishmania major. Mol Biochem Parasitol. 1994;65:259–270. doi: 10.1016/0166-6851(94)90077-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.