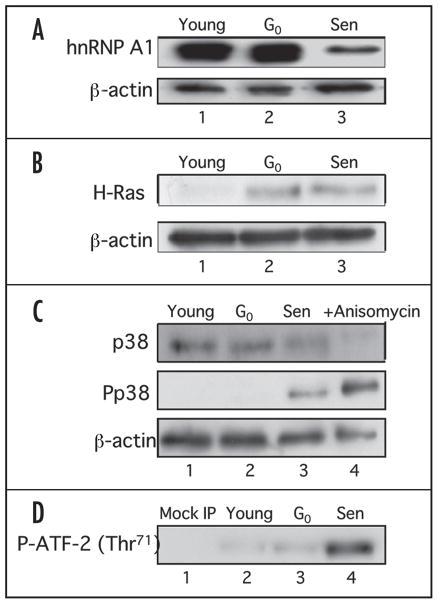
Figure 1.
Western analysis of hnRNP A1 and the Ras signaling pathway in IMR-90 young, G0-arrested and senescent cells. (A) Western analysis of hnRNP A1. 10 μg of whole cell lysates from young (lane 1), G0-arrested (lane 2) and senescent (lane 3) cells were electrophoresed on a 12% SDS-PAGE and probed with 4B10 monoclonal antibody specific for hnRNP A1. The blot was then stripped and reprobed to detect total β-actin levels. (B) Western analysis of c-H-Ras levels. Fifty micrograms of whole cell lysates from young (lane 1), G0-arrested (lane 2) and senescent (lane 3) cells were subjected to 15% SDS-PAGE and c-H-Ras levels were determined by immunoblot analysis using H-Ras antibody. The membrane was stripped and reprobed with β-actin antibody as a loading control. (C) Western analysis of p38 MAPK and phospho-p38 MAPK levels. 25 μg of whole cell lysates from young (lane 1), G0-arrested (lane 2) and senescent (lane 3) cells were electrophoresed on a 12% SDS-PAGE and probed with antibodies for p38 MAPK, phospho-p38 MAPK (P-p38 MAPK) and β-actin. Anisomycin treated lysates were used as positive controls (lane 4). (D) p38 MAPK activity in IMR-90 cells. 400 μg of whole cell lysates from young (lane 2), G0-arrested (lane 3) and senescent (lane 4) fibroblasts were incubated with immobilized anti-phosphorylated p38 MAPK beads and the kinase activity was measured using a fusion ATF-2 protein with a single phosphorylation site at Thr71. A mock IP reaction containing antibody and beads only was included as a negative control (lane 1).