Abstract
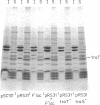
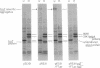
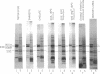
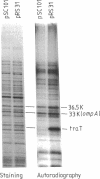
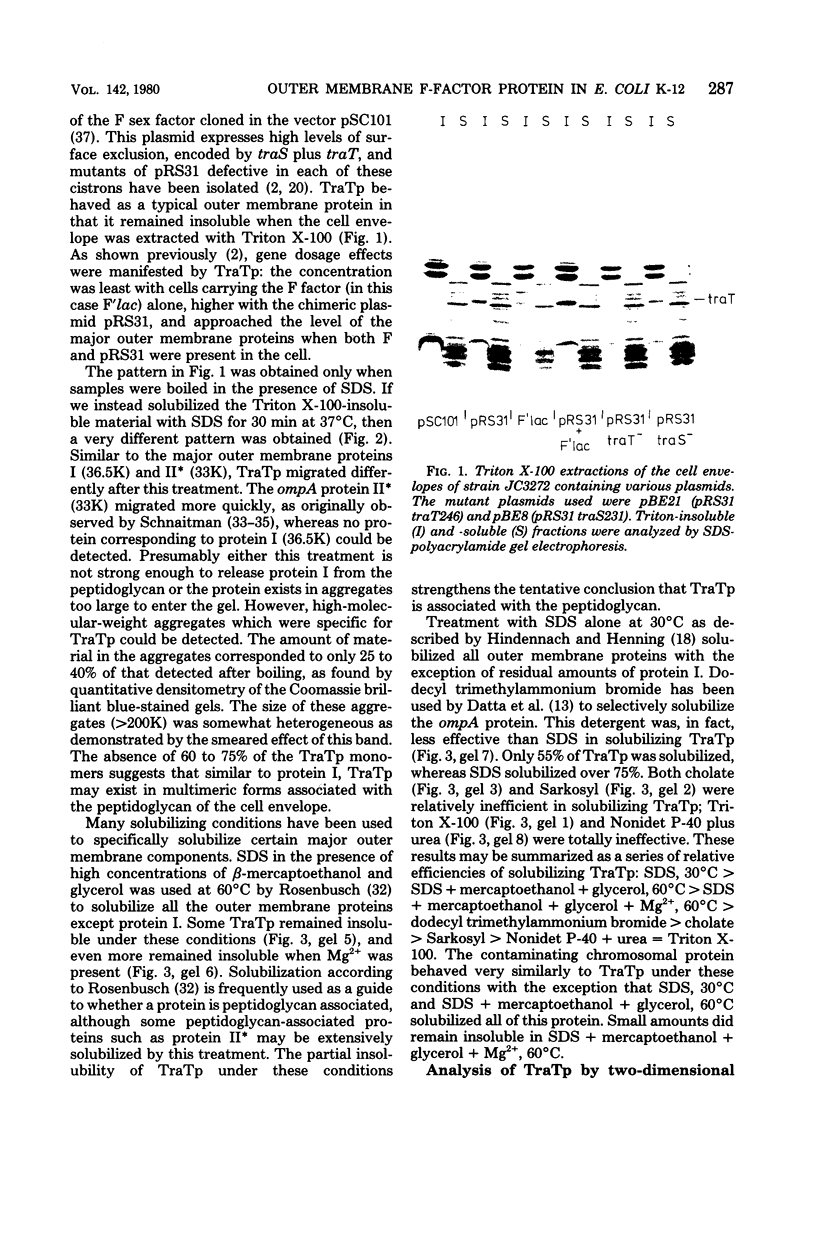
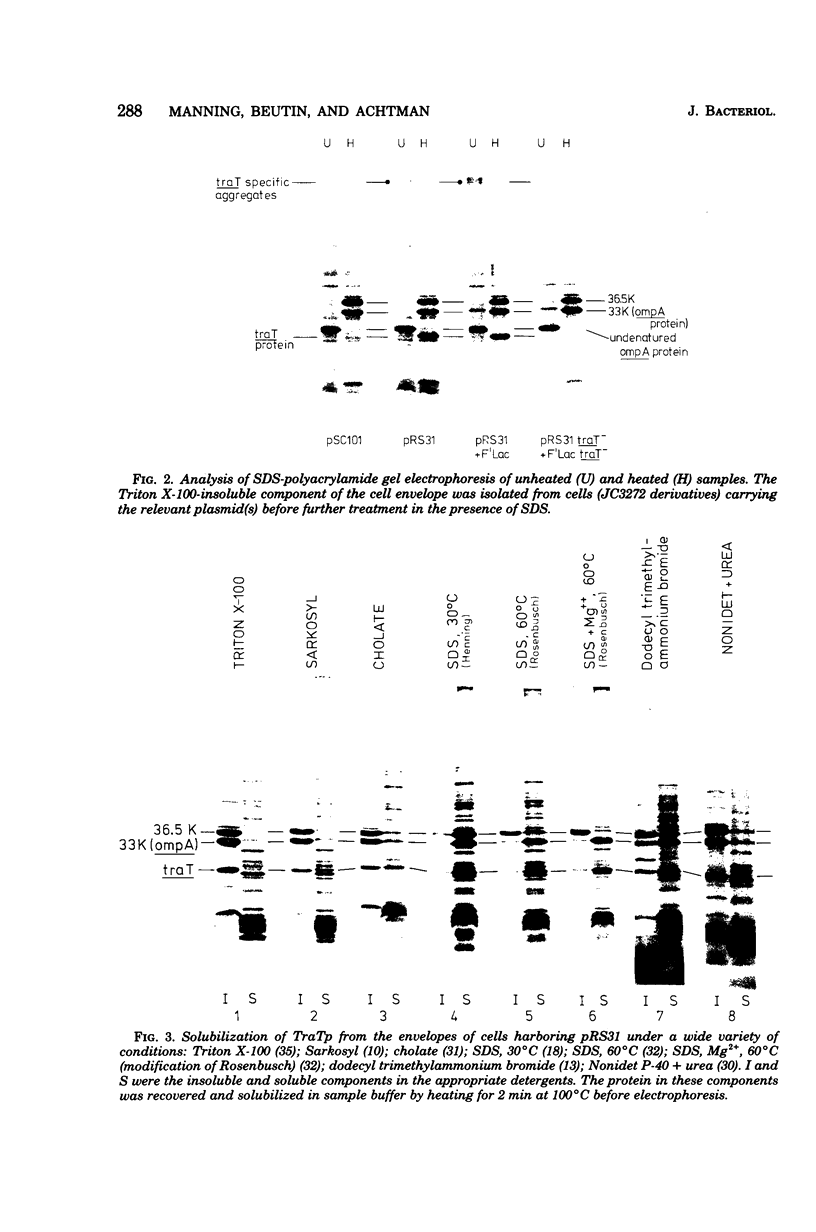
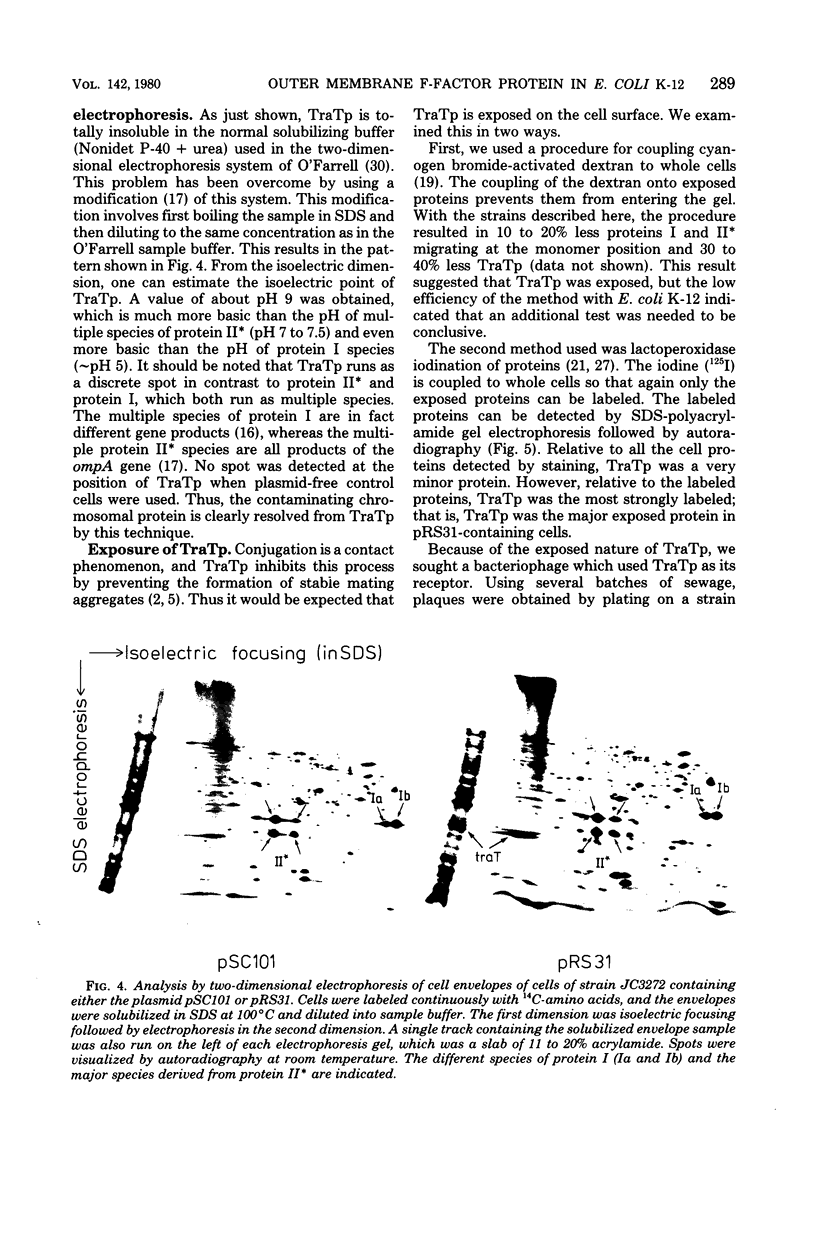
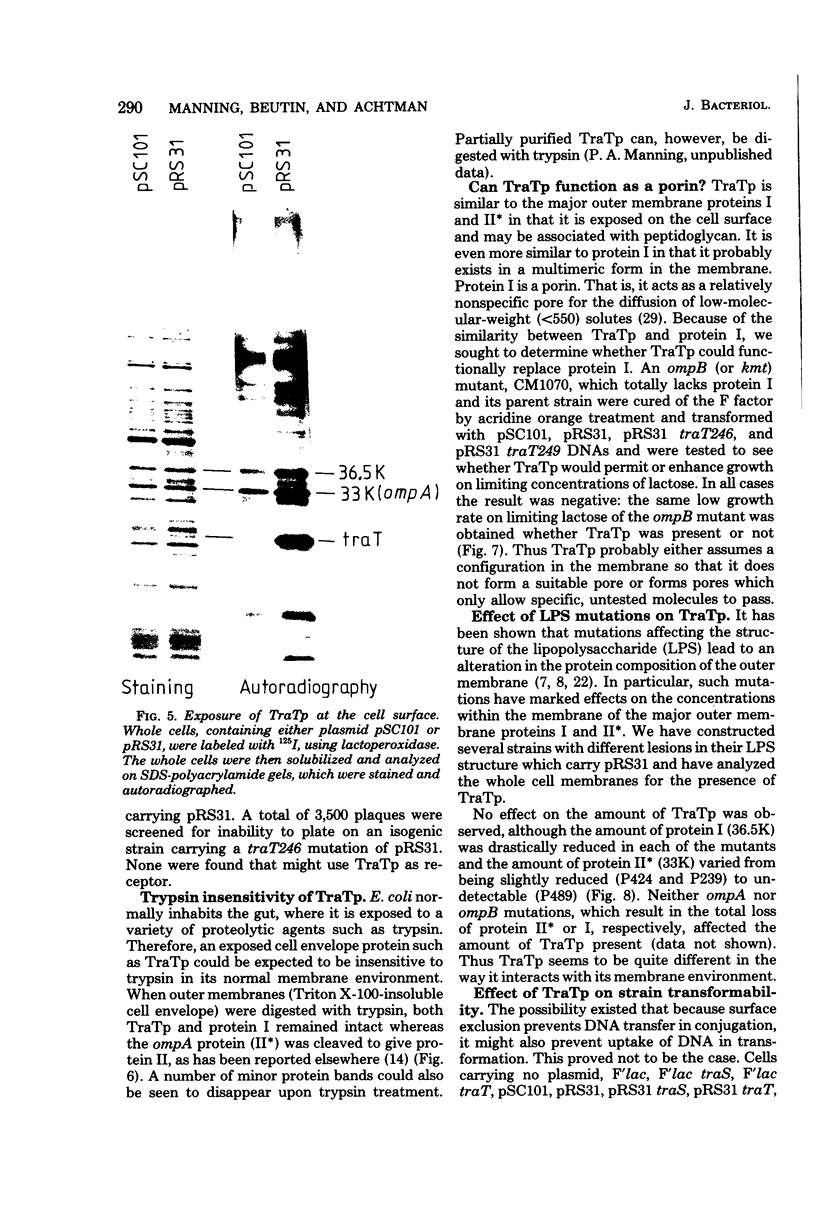
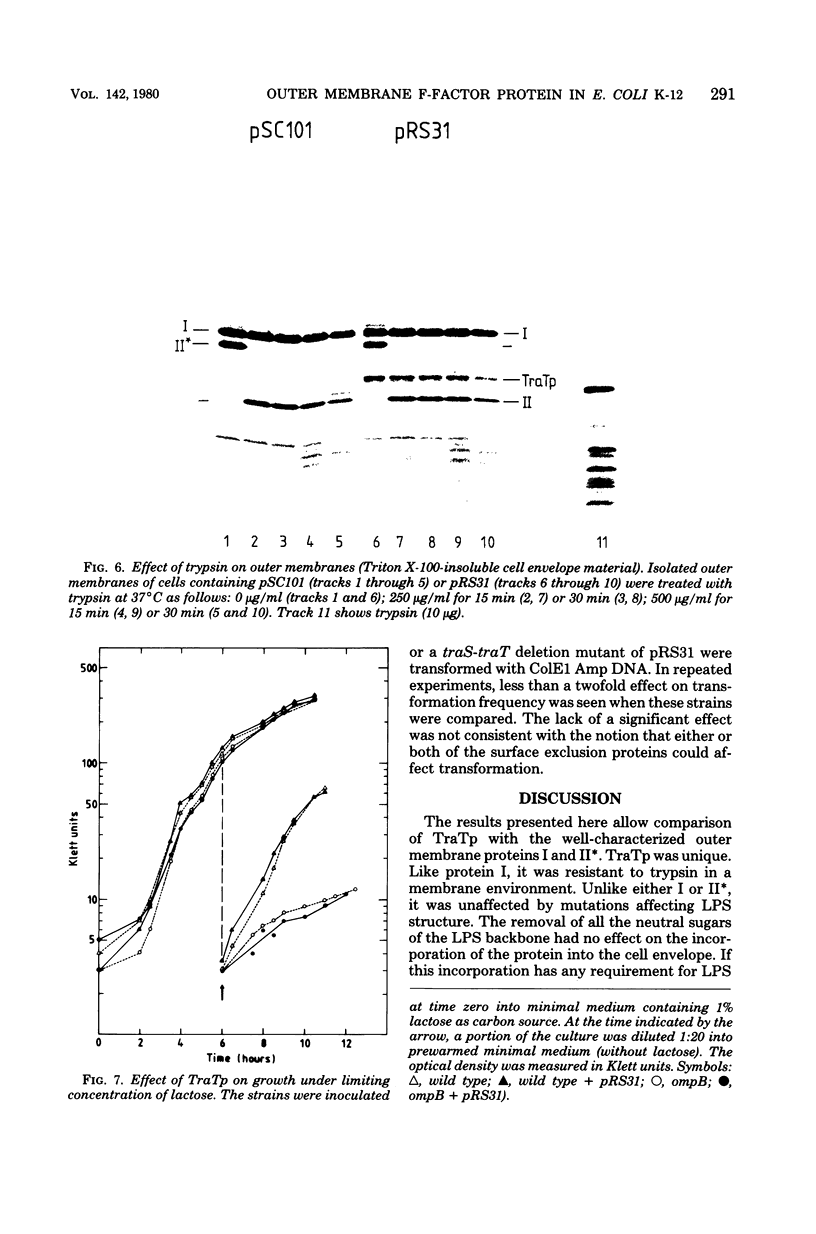
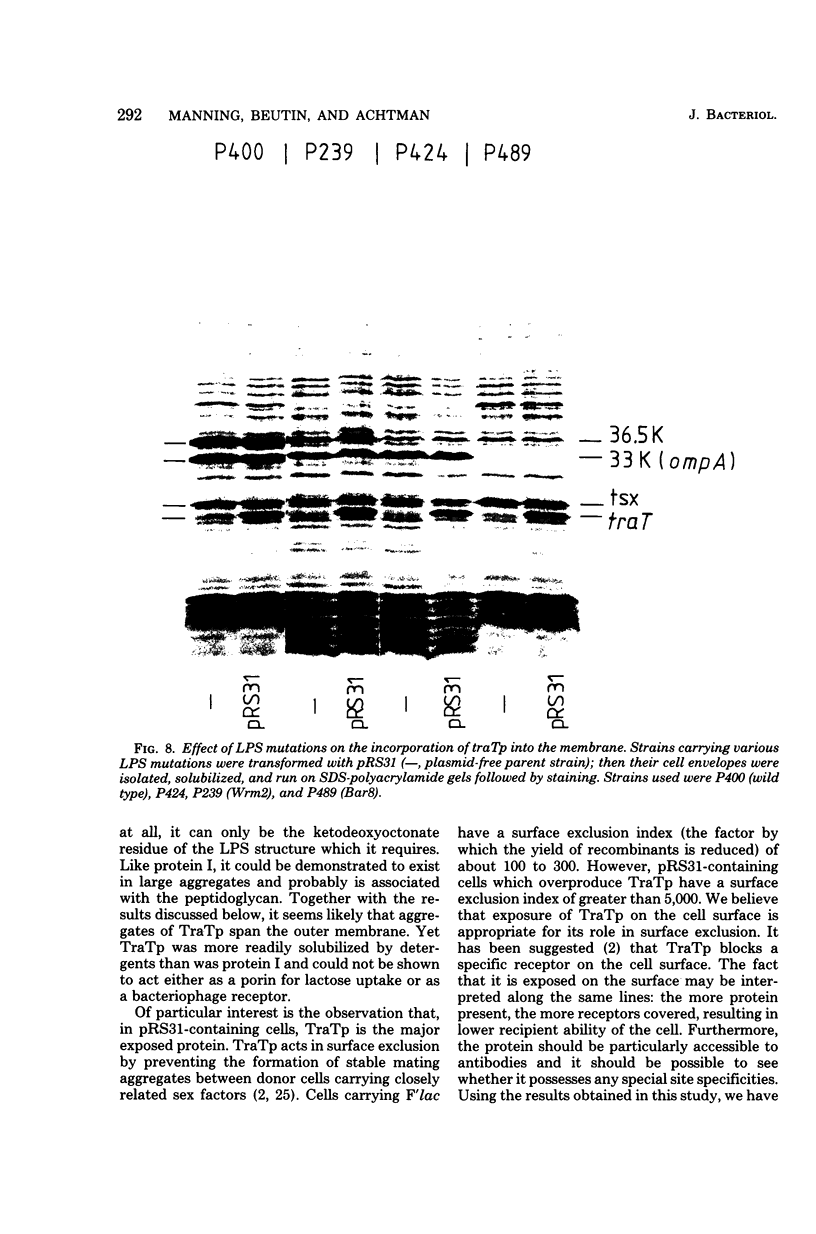
The traT protein (TraTp) of the F sex factor is the product of one of the two genes involved in surface exclusion. Several detergents were examined under different conditions in order to determine their ability to solubilize TraTp from membrane vesicles. These experiments showed that TraTp behaved similar to a number of peptidoglycan-associated outer membrane proteins and that it existed in multimeric aggregates within the membrane. However, unlike other major outer membrane proteins, the amount of TraTp incorporated into the membrane was not affected by lipopolysaccharide-deficient mutants, even when mutants totally lacking the neutral sugars in their lipopolysaccharide backbone were used. TraTp wqs also examined by two-dimensional gel electrophoresis, where it ran as a discrete spot with a very basic isoelectric point. By coupling cyanogen bromide-activated dextran onto whole cells and by labeling whole cells with 125I (via lactoperoxidase), it was shown that TraTp was exposed on the cell surface. TraTp in a membrane environment was also insensitive to proteolytic attack by trypsin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975 Aug;123(2):505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Holland I. B. Protein d, an iron-transport protein induced by filtration of cultures of Escherichia coli. FEBS Lett. 1977 Apr 1;76(1):20–24. doi: 10.1016/0014-5793(77)80112-9. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller I., Henning U. Cell envelope and shape of Escherichia coli K12. Crosslinking with dimethyl imidoesters of the whole cell wall. Proc Natl Acad Sci U S A. 1974 May;71(5):2018–2021. doi: 10.1073/pnas.71.5.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Lipopolysaccharide-deficient, bacteriophage-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):98–108. doi: 10.1128/jb.127.1.98-108.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Schmidmayr W., Hindennach I. Major proteins of the outer cell envelope membrane of Escherichia coli K-12: multiple species of protein I. Mol Gen Genet. 1977 Sep 9;154(3):293–298. doi: 10.1007/BF00571285. [DOI] [PubMed] [Google Scholar]
- Henning U., Sonntag I., Hindennach I. Mutants (ompA) affecting a major outer membrane protein of Escherichia coli K12. Eur J Biochem. 1978 Dec;92(2):491–498. doi: 10.1111/j.1432-1033.1978.tb12771.x. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J, Cavalli L L, Lederberg E M. Sex Compatibility in Escherichia Coli. Genetics. 1952 Nov;37(6):720–730. doi: 10.1093/genetics/37.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. II. Heterogeneity of major outer membrane polypeptides. Arch Biochem Biophys. 1973 Aug;157(2):553–560. doi: 10.1016/0003-9861(73)90674-7. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Maule J. Interactions between the surface exclusion systems of some F-like plasmids. Genet Res. 1974 Aug;24(1):81–89. doi: 10.1017/s0016672300015093. [DOI] [PubMed] [Google Scholar]
- van Alphen W., Lugtenberg B., Berendsen W. Heptose-deficient mutants of Escherichia coli K12 deficient in up to three major outer membrane proteins. Mol Gen Genet. 1976 Sep 23;147(3):263–269. doi: 10.1007/BF00582877. [DOI] [PubMed] [Google Scholar]
- von Meyenburg Kaspar Transport-limited growth rates in a mutant of Escherichia coli. J Bacteriol. 1971 Sep;107(3):878–888. doi: 10.1128/jb.107.3.878-888.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]