Abstract
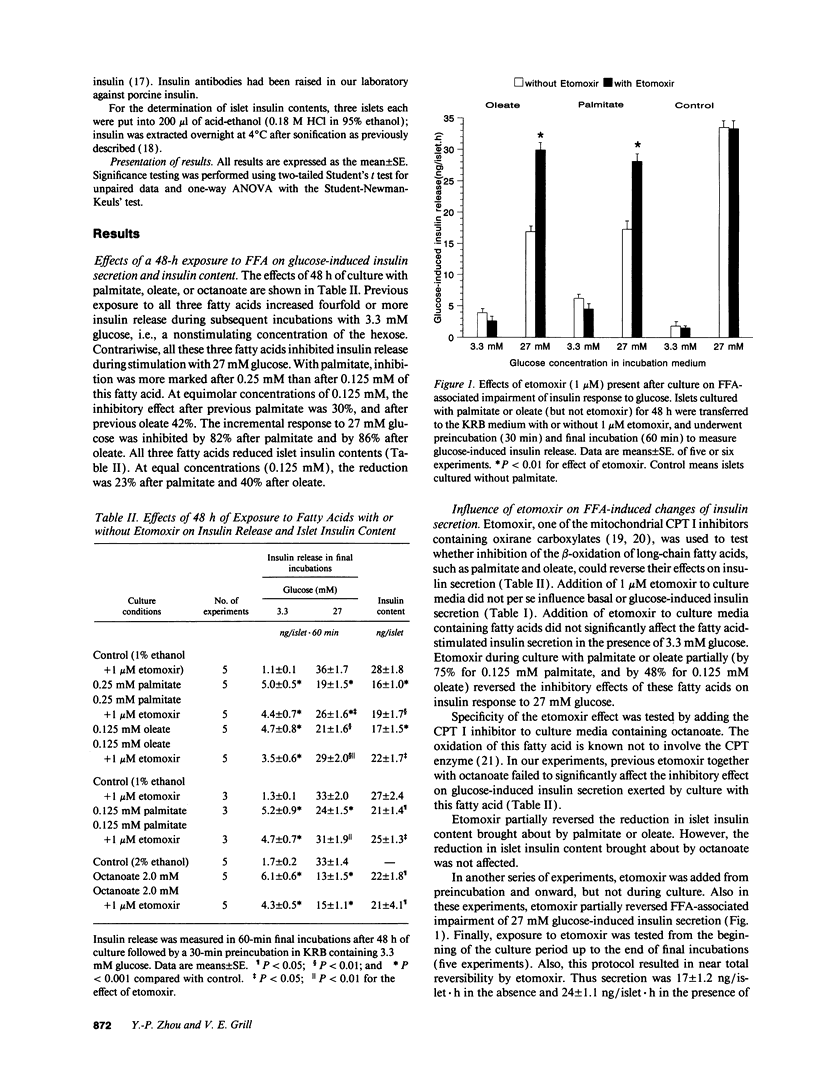
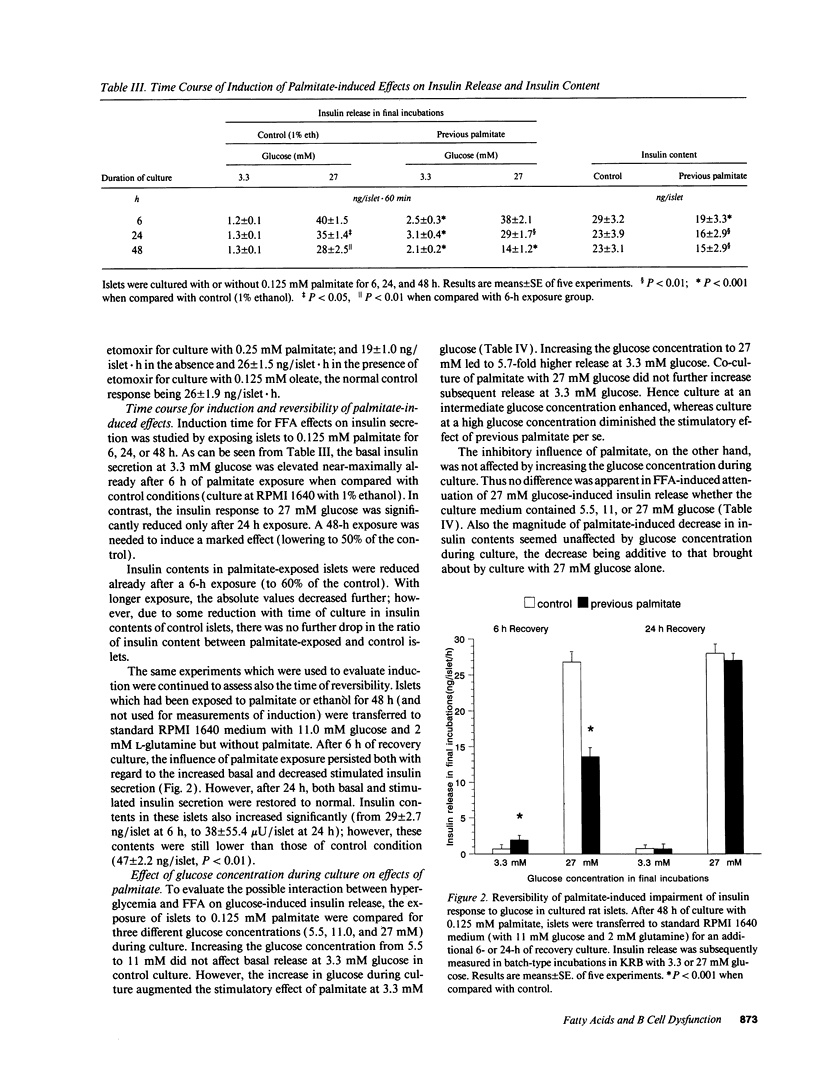
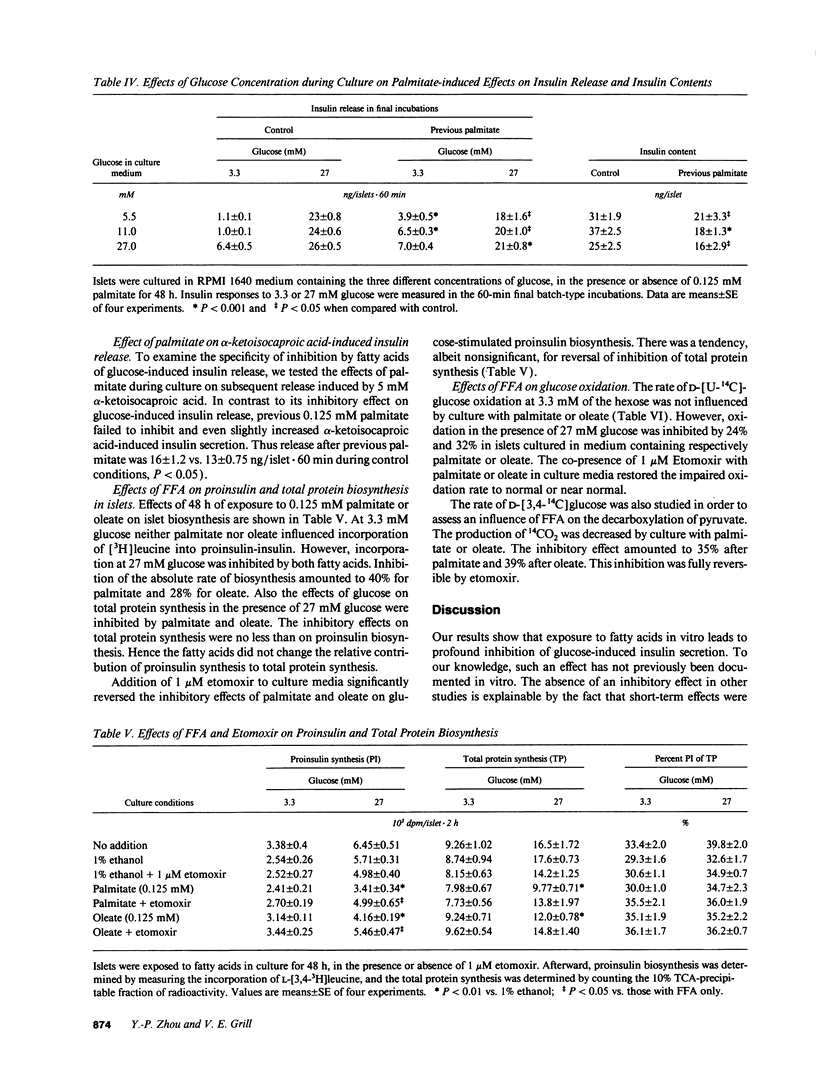
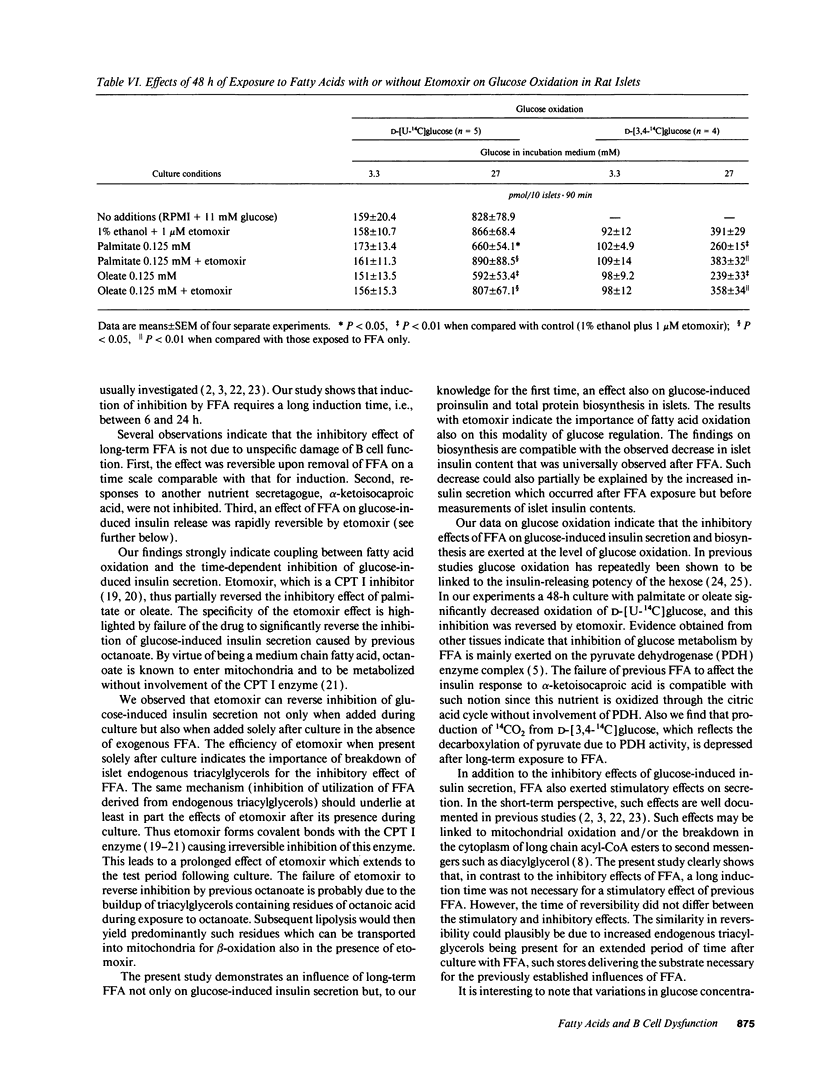
We tested effects of long-term exposure of pancreatic islets to free fatty acids (FFA) in vitro on B cell function. Islets isolated from male Sprague-Dawley rats were exposed to palmitate (0.125 or 0.25 mM), oleate (0.125 mM), or octanoate (2.0 mM) during culture. Insulin responses were subsequently tested in the absence of FFA. After a 48-h exposure to FFA, insulin secretion during basal glucose (3.3 mM) was several-fold increased. However, during stimulation with 27 mM glucose, secretion was inhibited by 30-50% and proinsulin biosynthesis by 30-40%. Total protein synthesis was similarly affected. Conversely, previous palmitate did not impair alpha-ketoisocaproic acid (5 mM)-induced insulin release. Induction and reversibility of the inhibitory effect on glucose-induced insulin secretion required between 6 and 24 h. Addition of the carnitine palmitoyltransferase I inhibitor etomoxir (1 microM) partially reversed (by > 50%) FFA-associated decrease in secretory as well as proinsulin biosynthetic responses to 27 mM glucose. The inhibitory effect of previous palmitate was similar when co-culture was performed with 5.5, 11, or 27 mM glucose. Exposure to palmitate or oleate reduced the production of 14CO2 from D-[U-14C]glucose, and of 14CO2 from D-[3,4-14C]-glucose, both effects being reversed by etomoxir. Conclusions: long-term exposure to FFA inhibits glucose-induced insulin secretion and biosynthesis probably through a glucose fatty acid cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berne C. The metabolism of lipids in mouse pancreatic islets. The oxidation of fatty acids and ketone bodies. Biochem J. 1975 Dec;152(3):661–666. doi: 10.1042/bj1520661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss C. R., Sharp G. W. Glucose-induced insulin release in islets of young rats: time-dependent potentiation and effects of 2-bromostearate. Am J Physiol. 1992 Nov;263(5 Pt 1):E890–E896. doi: 10.1152/ajpendo.1992.263.5.E890. [DOI] [PubMed] [Google Scholar]
- Campillo J. E., Valdivia M. M., Rodriguez E., Osorio C. Effect of oleic and octanoic acids on glucose-induced insulin release in vitro. Diabete Metab. 1979 Sep;5(3):183–187. [PubMed] [Google Scholar]
- Capito K., Hansen S. E., Hedeskov C. J., Islin H., Thams P. Fat-induced changes in mouse pancreatic islet insulin secretion, insulin biosynthesis and glucose metabolism. Acta Diabetol. 1992;28(3-4):193–198. doi: 10.1007/BF00778997. [DOI] [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Stimulation of insulin secretion by long-chain free fatty acids. A direct pancreatic effect. J Clin Invest. 1973 Aug;52(8):1979–1984. doi: 10.1172/JCI107382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq P. E., Falck J. R., Kuwajima M., Tyminski H., Foster D. W., McGarry J. D. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. I. Use of inhibitors. J Biol Chem. 1987 Jul 15;262(20):9812–9821. [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E. Rationale and application of fatty acid oxidation inhibitors in treatment of diabetes mellitus. Diabetes Care. 1992 Jun;15(6):773–784. doi: 10.2337/diacare.15.6.773. [DOI] [PubMed] [Google Scholar]
- Goberna R., Tamarit J., Jr, Osorio J., Fussgänger R., Tamarit J., Pfeiffer E. F. Action of B-hydroxy butyrate, acetoacetate and palmitate on the insulin release in the perfused isolated rat pancreas. Horm Metab Res. 1974 Jul;6(4):256–260. doi: 10.1055/s-0028-1093862. [DOI] [PubMed] [Google Scholar]
- Grill V., Rundfeldt M., Efendić S. Previous exposure to glucose enhances somatostatin secretion from the isolated perfused rat pancreas. Diabetologia. 1981 Apr;20(4):495–500. doi: 10.1007/BF00253414. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B., Blondel B., Renold A. E. Long-term exposure of isolated pancreatic islets to mannoheptulose: evidence for insulin degradation in the beta cell. Biochem Pharmacol. 1980 Oct 1;29(19):2625–2633. doi: 10.1016/0006-2952(80)90077-5. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- KEEN H., FIELD J. B., PASTAN I. H. A simple method for in vitro metabolic studies using small volumes of tissue and medium. Metabolism. 1963 Feb;12:143–147. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Best L., Kawazu S., Malaisse-Lagae F., Sener A. The stimulus-secretion coupling of glucose-induced insulin release: fuel metabolism in islets deprived of exogenous nutrient. Arch Biochem Biophys. 1983 Jul 1;224(1):102–110. doi: 10.1016/0003-9861(83)90193-5. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Insulin release : the fuel concept. Diabete Metab. 1983 Dec;9(4):313–320. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Sener A., Hellerström C. Participation of endogenous fatty acids in the secretory activity of the pancreatic B-cell. Biochem J. 1985 May 1;227(3):995–1002. doi: 10.1042/bj2270995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. Stimulation of insulin secretion by noncarbohydrate metabolites. J Lab Clin Med. 1968 Sep;72(3):438–448. [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Prentki M., Vischer S., Glennon M. C., Regazzi R., Deeney J. T., Corkey B. E. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem. 1992 Mar 25;267(9):5802–5810. [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Kerbey A. L., Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988 Nov;4(7):623–638. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
- Sako Y., Grill V. E. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990 Oct;127(4):1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. Stimulation by D-glucose of mitochondrial oxidative events in islet cells. Biochem J. 1987 Aug 15;246(1):89–95. doi: 10.1042/bj2460089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarit-Rodríguez J., Vara E., Tamarit J. Starvation-induced changes of palmitate metabolism and insulin secretion in isolated rat islets stimulated by glucose. Biochem J. 1984 Jul 15;221(2):317–324. doi: 10.1042/bj2210317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H. P., Eistetter K., Ludwig G. Phenylalkyloxirane carboxylic acids, a new class of hypoglycaemic substances: hypoglycaemic and hypoketonaemic effects of sodium 2-[5-(4-chlorophenyl)-pentyl]-oxirane-2-carboxylate (B 807-27) in fasted animals. Diabetologia. 1982 Jun;22(6):456–463. doi: 10.1007/BF00282590. [DOI] [PubMed] [Google Scholar]


