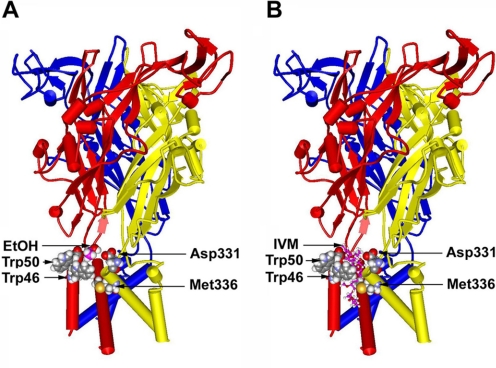
Fig. 8.
Molecular model of the rat P2X4R built by threading the edited primary sequence onto the X-ray crystal structure of zebrafish P2X4R. A, a side view of the rat P2X4R showing the ectodomain and the six α-helices of TM1 and TM2 segments of three different P2X4R subunits with a single ethanol molecule manually inserted at the interface. All residues of interest were rendered as space-filling surfaces; the ethanol molecule is colored pink to distinguish it. Residues Trp46 and Trp50 in the first α-helix of one subunit and Asp331 and Met336 in the final α-helix of the adjacent subunit form a pocket that demonstrates a good fit for a molecule of ethanol at the same scale. B, a similar view of the rat P2X4R, but with a model of IVM (rendered in balls and sticks) inserted into a putative binding site in a position between the α-helices such as that described in nicotinic acetylcholine receptors (Sattelle et al., 2009).