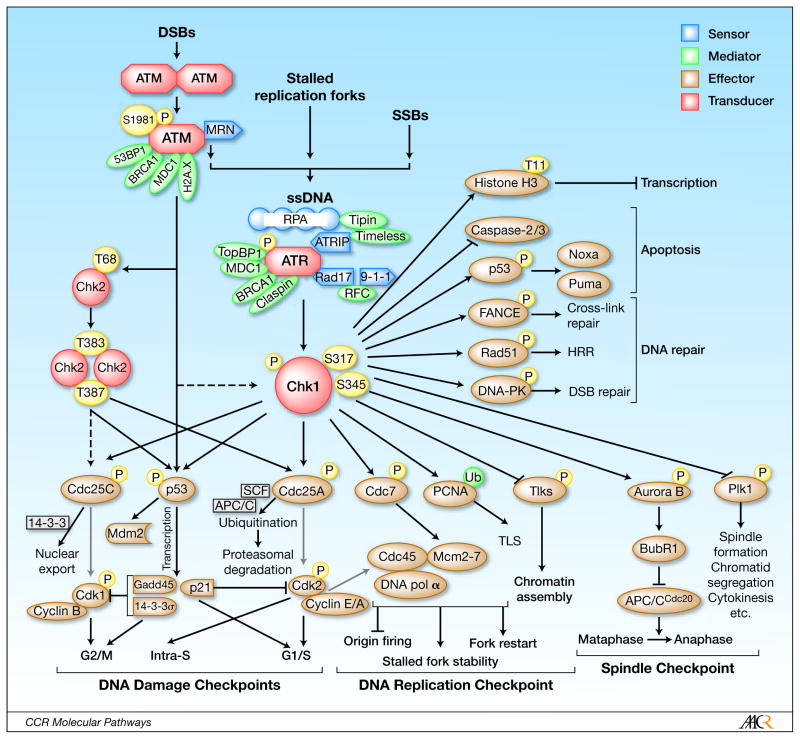
Figure 1. Chk1 in DDR signaling network.
DNA damage (e.g., double strand breaks/DSBs, single-strand breaks/SSBs, and stalled replication forks) generates single-strand DNA (ssDNA) that initiates ATR-mediated Chk1 activation. In this context, the ATR/ATPIP complex is recruited to ssDNA lesions via binding of ATRIP with RPA that recognizes and coats ssDNA. In conjunction with recruited/activated “sensors” and “mediators”, ATR phosphorylates Chk1 at two canonical sites (Ser345 and S317), directly leading to its activation without the homodimerization and intramolecular trans-autophosphorylation that is required for Chk2 activation. Activated Chk1 then phosphorylates diverse downstream “effectors”, which in turn are involved in cell cycle checkpoints (i.e., intra-S-, G2/M-, and G1/S-phase checkpoints), the DNA replication checkpoint, the mitotic spindle checkpoint, as well as DNA repair, apoptosis, and transcription. Consequently, Chk1 is a kinase central for the DDR signaling network, thereby representing a particularly attractive target in anti-cancer therapeutics.