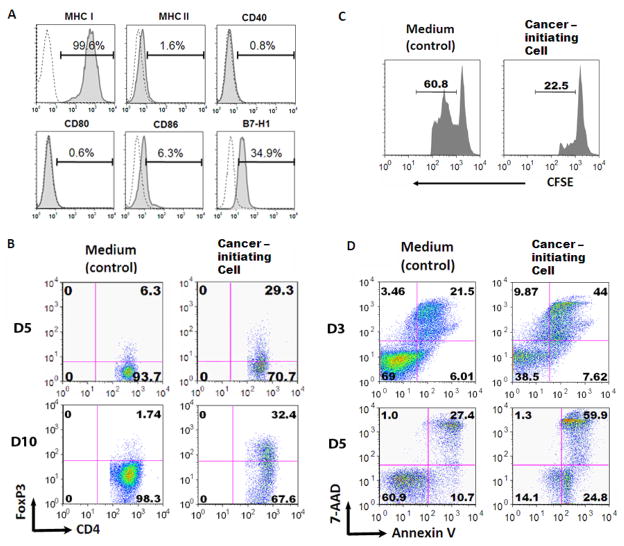
Figure 2. Glioma-associated cancer-initiating cells are immunosuppressive of human T cells.
A. Immune surface phenotype of a representative glioma-associated cancer-initiating cell. The glioma-associated cancer-initiating cells were surface stained with antibodies to MHC I, MHC II, CD40, CD80, CD86, and B7-H1. Representative FACS histogram plots for one glioma-associated cancer-initiating cell are shown for target staining (solid line) with associated isotype controls (dotted line). Percentages of the positive populations are shown. B. The supernatants from the glioma-associated cancer-initiating cells induce an increase in the number of CD4+FoxP3+ Tregs on both day 5 and 10. Representative FACS plots are shown. C. The glioma-associated cancer-initiating cell induced FoxP3+ Tregs suppress T cell proliferation. T cells that were treated with glioma-associated cancer-initiating cell supernatants were harvested, co-cultured for 3 days with autologous PBMCs (labeled with CFSE, responder cells) at a 1:1 ratio in the presence of soluble anti-CD3 and subsequently analyzed via FACScan. The number above the line in each histogram represents proliferating responder cells. D. The glioma-associated cancer-initiating cell supernatants induce T cell apoptosis after 3 days of exposure to the supernatants. Similar data was obtained after 5 days of exposure. After culturing with the glioma-associated cancer-initiating cell supernatants, T cells were stimulated with anti-CD3/CD28 and stained with 7-AAD and Annexin V. Compared to medium alone (control), the glioma-associated cancer-initiating cells enhanced T cell apoptosis.