Abstract
BACKGROUND
The LH surge promotes ovulation via activation of multiple signaling networks in the ovarian follicle. Studies in animal models have shown the importance of LH-induced activation of the epidermal growth factor (EGF)signaling network in critical peri-ovulatory events. We investigated the biological significance of regulatory mechanisms mediated by EGF-like growth factors during LH stimulation in humans.
METHODS
We characterized the EGF signaling network in mature human ovarian follicles using in vivo and in vitro approaches. Amphiregulin (AREG) levels were measured in 119 follicular fluid (FF) samples from IVF/ICSI patients. Biological activity of human FF was assessed using in vitro oocyte maturation, cumulus expansion and cell mitogenic assays.
RESULTS
AREG is the most abundant EGF-like growth factor accumulating in the FF of mature follicles of hCG-stimulated patients. No AREG was detected before the LH surge or before hCG stimulation of granulosa cells in vitro, demonstrating that the accumulation of AREG requires gonadotrophin stimulation. Epiregulin and betacellulin mRNA were detected in both human mural and cumulus granulosa cells, although at significantly lower levels than AREG. FF from stimulated follicles causes cumulus expansion and oocyte maturation in a reconstitution assay. Immunodepletion of AREG abolishes the ability of FF to stimulate expansion (P < 0.0001) and oocyte maturation (P < 0.05), confirming the biological activity of AREG. Conversely, mitogenic activity of FF remained after depletion of AREG, indicating that other mitogens accumulate in FF. FF from follicles yielding an immature germinal vesicle oocyte or from an oocyte that develops into an aberrant embryo contains lower AREG levels than that from follicles yielding a healthy oocyte (P = 0.008).
CONCLUSIONS
EGF-like growth factors play a role in critical peri-ovulatory events in humans, and AREG accumulation is a useful marker of gonadotrophin stimulation and oocyte competence.
Keywords: epidermal growth factor, gonadotrophin, folliculogenesis, ovary, amphiregulin
Introduction
Oocyte re-entry into the meiotic cell cycle and completion of maturation, as well as activation of two distinct programs of terminal differentiation in mural granulosa cells (mGCs) and cumulus cells (CCs) surrounding the oocyte, pave the way to the ovulatory process. These events are followed by the rupture of the follicle and extrusion of a mature cumulus–oocyte complex (COC) competent for fertilization. All the events are triggered by a rapid increase in circulating levels of the gonadotrophin LH. Although critical for fertility, the precise roles of LH in promoting the final stages of oocyte maturation are incompletely understood. LH signaling is crucial for the final stages of oocyte maturation (Chandrasekher et al., 1994; Sullivan et al., 1999; Hillier, 2001). However, LH/hCG receptors are present on theca, on mGCs as well as on cumulus granulosa cells in some species, but not on the oocyte itself. The canonical GH-mediated cAMP pathway is activated by LH receptor signaling, but cAMP levels are not the only mechanism by which LH exerts its effects on oocyte maturation. Recent studies have shown that LH signaling at the time of final oocyte maturation involves activation of the small G protein Ras as well as activation of the mitogen-activated protein kinases ERK1/2, although the precise mechanisms by which the LH receptor signals in this manner have not yet been fully elucidated (Fan et al., 2008, 2009).
In multiple species, including mouse, rat, pig, cow and rhesus monkey, LH receptor activation causes up-regulation of the epidermal growth factor (EGF)-like growth factor family (Ashkenazi et al., 2005; Fru et al., 2007; Yamashita et al., 2007; Li et al., 2009). This is a potential mechanism for transducing the LH signal to the oocyte, since these growth factors are known to interact with the CCs which are in intimate contact with the developing oocyte.
In the mouse, amphiregulin (AREG), epiregulin (EREG) and betacellulin (BTC) have been show to be mediators of LH signaling in the follicle (Park et al., 2004). Further work has shown an impairment in female mouse fertility following genetic disruption or pharmacological blockade of LH-mediated transactivation of the EGFsignaling network (Hsieh et al., 2007). However, there does appear to be redundancy in this system since the AREG and EREG knockout mice are fertile, although with a decreased litter size compared with the wild type (Lee et al., 2004). Previous work in human follicular fluid (FF) has shown an EGF-like activity, although only very small amounts of EGF protein were found (Westergaard and Andersen, 1989; Reeka et al., 1998). Furthermore, microarray analysis has shown that primary human granulosa cultures do not show modulation of EGF expression by either LH or FSH (Freimann et al., 2004). This discrepancy may be explained by the presence of EGF-like ligands in human FF, which signal by binding to members of the EGF receptor family but do not cross-react with antibodies directed at EGF itself.
The three EGF-like ligands AREG, EREG and BTC were discovered within the past two decades (Shoyab et al., 1988; Shing et al., 1993; Toyoda et al., 1995). AREG and the other family members are synthesized as transmembrane precursors which are then cleaved to release the active growth factor which can interact with different members of the EGF receptor family (Sanderson et al., 2006). One prior study has examined the expression of an EGF-like growth factor, AREG, in FF (Inoue et al., 2009), and AREG was found in human FF obtained at the time of oocyte retrieval (OR) at significantly higher levels than either EGF or transforming growth factor-α. There was a non-significant trend towards higher AREG levels and poor quality embryos, and a potential association of higher AREG and lower fertilization rates. However, a limitation of the study of Inoue et al. (2009) is the use of pooled follicular samples which prevents any correlations with outcomes of individual oocytes or embryos.
The purpose of our study is to provide a detailed characterization of EGF-like growth factors in human FF and their regulation. Here, we provide evidence that bioactive AREG accumulation is induced by hCG and that levels of AREG in FF correlate with oocyte maturation.
Materials and Methods
Source and collection of human FF samples
Patients undergoing assisted reproduction treatments by standard ovarian stimulation protocols were recruited to collect FF. For this FF database, each patient had either one or two follicle aspirates collected at the time of OR 36 h after hCG from mature-sized follicles (≥16 mm diameter). For this particular study, only patients receiving down-regulated ovarian stimulation protocols were included and only a single FF sample from each patient was used. Details regarding the stimulation parameters, OR and FF preparation and storage have been described previously (Rosen et al., 2009). Additional information on the stimulation parameters is given below. Pre-hCG FF was obtained from mature-sized follicles (≥16 mm diameter). Five samples taken before hCG administration (pre-hCG samples) were obtained from the following types of patients: one ovum donor who did not inject hCG, two stimulated IVF patients with single lead follicles that were aspirated to allow salvage of ovarian stimulation, one stimulated patient undergoing fertility preservation enrolled in a clinical trial, and one normally cycling unstimulated patient undergoing laparoscopic tubal ligation who had a dominant follicle aspirated with a documented negative serum LH. mGCs were obtained by aspiration from FF after microscopic identification. CCs were obtained by mechanically separating a portion of the cumulus from the oocytes at the time of ICSI. The decision for conventional insemination or ICSI fertilization was made by the clinician based on the presence of a component of male factor infertility. The study was approved by the institutional review board at University of California, San Francisco (UCSF) and informed consent was obtained from all patients involved in this study.
Detailed ovarian stimulation protocol
For this study, we only used FF samples from patients fulfilling certain criteria. The stimulations involved only standard OCP-long luteal protocols for stimulation. Gonadotrophin doses and tapering during stimulation are based on a pre-determined clinical algorithm based on antral follicle count (AFC) and serum estradiol (E2) with all patients being monitored at the UCSF IVF Clinic. The dose of hCG for the samples used was fixed at 10 000 IU, given 36 h prior to OR. For all our comparisons, samples were age matched to the extent this was possible. For the germinal vesicle (GV) oocytes, there were fewer numbers therefore strict age matching was not possible; however, the average ages were not significantly different from the other groups. ICSI was used only for male factor infertility indications.
Specific long luteal protocol doses:
Starting gonadotrophin dose (mixed recombinant FSH and hMG): (150–450 IU) per day
AFC >20: 150/75
AFC 13–20: 150/150
AFC 8–12: 225/150
AFC <8: 300/150
The dose-tapering protocol is as follows. Day 4 E2 ≥ 150 pg/ml: decrease gonadotrophin by 75 IU of FSH product per day. Day 6 E2 second taper: E2 ≥ 350 pg/ml: decrease by additional 75 IU of FSH product per day.
Granulosa cell RNA extraction and quantitative RT–PCR
Total RNA from CCs or mGCs was isolated using the RNeasy Micro Kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. RNA quality was determined with the Bioanalyzer 2100 and RNA 6000 Pico LabChip assay (Agilent Technologies Inc., Palo Alto, CA, USA). Samples were reversed transcribed using an AffinityScript qPCR cDNA synthesis kit (Stratagene, La Jolla, CA, USA) with random hexamers. Real-time PCR was performed to quantify the expression levels of EGF-like growth factors using the SYBR GreenER qPCR SuperMix system (Invitrogen, Carlsbad, CA, USA). All primers were designed to span introns to ensure specific amplification of cDNA (Table I). To compare expression levels of different genes, the efficiency of each set of primers was calculated with a standard curve of serial dilutions, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize all the data.
Table I.
Primers used for quantitative RT–PCR analysis of human ovary and amplicon size.
| Ref Seq | Sense primer sequence 5′–3′ | Antisense primer sequence 5′–3′ | Amplicon size (bp) | |
|---|---|---|---|---|
| AREG | NM_001657.2 | AGCCGACTATGACTACTCAG | CTTAACTACCTGTTCAACTCTGAC | 96 |
| EREG | NM_001432.2 | GCACAGCTTTAGTTCAGACAG | TGTCCACCAGATAGATGCAC | 96 |
| BTC | NM_001729.2 | AAAGGCCACTTCTCTAGGT | CCTTCATCACAGACACAGG | 107 |
| ErbB1 | NM_005228.3 | GAGGATGTTCAATAACTGTGAGG | CCTGGATGGTCTTTAAGAAGG | 95 |
| ErbB2 | NM_004448.2 | GCCTGTCCCTACAACTACC | TGTTCCATCCTCTGCTGTC | 92 |
| ErbB3 | NM_001982.2 | TTCCTGCAGTGGATTCGAG | CAAACTTCCCATCGTAGACC | 120 |
| ErbB4 | NM_005235.2 | CTTGACAGAAATCCTAAATGGTGG | GTCCACATCCTGAACTACCA | 148 |
| GAPDH | NM_002046.3 | CATTTCCTGGTATGACAACGA | TTCCTCTTGTGCTCTTGCT | 101 |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Quantification of EGF-like growth factor levels
AREG protein levels were quantified using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer's instructions. Serial dilutions were used with a minimum of two points within the standard curve used for each sample. Each dilution was run in duplicate, with 9% inter-assay coefficient of variation. BTC levels were quantified using a commercially available ELISA (R&D Systems Inc.). EREG levels were quantified by ELISA using a mouse monoclonal anti-human primary antibody and a biotinylated goat anti-human secondary antibody with similar conditions to the AREG ELISA (R&D Systems Inc.).
Immunodepletion of human FF
Immunodepletion of FF was performed by incubating FF supplemented with protease inhibitors (Complete Mini, Roche, Basel, Switzerland) and either goat immunoglobulin (Ig)G anti-human AREG or goat IgG anti-human EREG antibodies (R&D Systems Inc.) or control IgG and Protein A/G beads, and tumbling overnight at 4°C. The supernatant was used for subsequent experiments. For the AREG immunodepletions, the supernatants were assayed by ELISA and showed >95% AREG removal.
COC expansion assay
COCs were isolated 44–48 h post-pregnant mare's serum gonadotrophin (PMSG) injection, as previously described, from C57BL/6 mice and were cultured in minimum essential medium (MEM, Gibco, Grand Island, NY, USA) supplemented with penicillin, streptomycin and 5% fetal bovine serum (Su et al., 2002). COCs were cultured at 37°C in 5% CO2. Recombinant mouse AREG (100 nM) (R&D Systems Inc.) and 10% human FF were added as controls. After culture for 16–18 h with the appropriate FF sample, COCs were scored for cumulus expansion by quantification of COC area using QCapture Pro Version 5.1 for Windows (Media Cybernetics Inc., Bethesda, MD, USA). Data are presented as the mean ± SEM of nine or more COCs analyzed in three separate experiments.
Oocyte maturation assay
COCs were isolated 44–48 h post-PMSG injection, as described above. The COCs were then incubated for 5–5.5 h at 37°C in MEM supplemented with 3.85 mM hypoxanthine and 10% human FF where indicated. The oocytes were then mechanically denuded by pipet and assessed for nuclear maturation by microscopy.
Mitogenic assay
Mouse Balb 3T3 cells were used because of their ability to respond to recombinant human AREG (Adam et al., 1999). Cells were grown to ∼30% confluence in 12-well dishes and serum starved for 6–8 h. Medium supplemented with appropriate FF fractions to a final concentration of 2.5% was then placed on the cells. [3H] Thymidine (0.4 µCi/well, 20 Ci/mmol; Perkin Elmer) was added to each well for ∼36 h. Cells were washed in medium and lysed in 0.2 N NaOH and 1% sodium dodecyl sulphate. [3H] Thymidine incorporation was determined by liquid scintillation counting. Results are expressed as pmol [3H] Thymidine over unstimulated controls. All samples were assayed in duplicate, and all values are expressed as mean ± SEM, with minimum n = 3.
Analysis of FF by chromatography
Pooled FF obtained from an ovum donor 36 h after hCG administration was clarified by centrifugation at room temperature for 10 min at 1500g, aliquoted into 2 ml cryovials, and placed at −80°C for later analysis. This fluid was thawed, diluted 1:8 with 20 mm Tris pH 8.5 and a cocktail of protease inhibitors was added (Complete Mini, Roche, Basel, Switzerland). The FF was then centrifuged at 4°C for 15 min at 15 000g and the supernatant loaded onto a Mono-Q column. Column parameters: bed volume 1 ml, flow rate 1 ml/min, fraction volume 0.6 ml. Low salt buffer 20 mm Tris pH 8.5, 10 mM NaCl. High salt buffer 20 mm Tris pH 8.5, 1 M NaCl. Elution gradient: low salt buffer × 10 min, then linear gradient to 100% high salt buffer over 40 min. Fractions were pooled in groups of three and assayed for AREG by ELISA, and total protein concentration by bicinchoninic acid (BCA) protein assay and OD280. Fractionation was repeated for fluid from a different ovum donor, giving a similar column elution profile.
Statistical analyses
Analysis of variance (ANOVA) was performed to compare levels of AREG between multiple groups. The mitogenic [3H] Thymidine results were analyzed by ANOVA with comparison t-tests where appropriate. Where the ANOVA was significant, Student's t-test with Bonferroni correction was performed for pairwise comparisons between groups. Logistic regression analyses were used to determine any significant relationship between levels of AREG and other relevant clinical parameters (female age, follicular size, follicular hCG). Tests were declared statistically significant for a two-sided P-value <0.05. All analyses were performed using Graphpad PRISM version 5.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
EGF-like growth factors are present in human FF after hCG stimulation
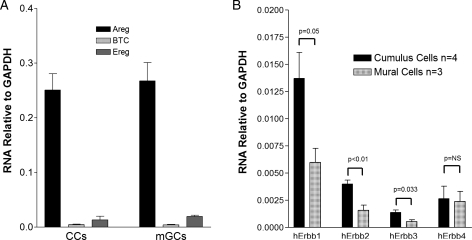
CCs and mGCs were isolated from the FF of women undergoing OR for IVF. We examined the expression of three EGF-like growth factors, AREG, EREG and BTC, by quantitative RT–PCR, and the results are summarized in Fig. 1A. Expression of mRNAs coding for all three growth factors was detected in both CCs and mGCs. With the caveat of potential differences in amplification efficiency, Areg mRNA was 10-fold higher than Ereg mRNA and 60-fold higher than BTC mRNA (P < 0.001). When the mRNA levels of Erbb1, the receptor shared by these three factors, were measured, higher concentrations were detected in CCs compared with mGCs, confirming that the CCs are a sensitive target for EGF-like growth factors (Fig. 1B).
Figure 1.
Expression of EGF-like growth factors and cognate receptors in the human ovarian follicle. Mural (mGC) and cumulus (CC) granulosa cells were collected 36 h after hCG administration. Total RNA was then isolated and RT–PCR performed. Results are reported as the mean ± SEM of three different samples for EGF-like growth factor expression and three to four different samples for EGF receptor (Erbb) subtype expression. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
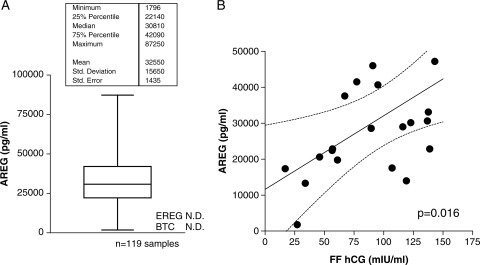
We next evaluated the protein levels of these three EGF-like ligands in human FF. High levels of AREG protein were observed in both pooled FF and individual follicle aspirates. AREG concentration ranged from ∼20 000 to 80 000 pg/ml with a distribution that was not significantly different from normal (Fig. 2A). To confirm the specificity of AREG detection, we immunodepleted multiple FF samples with an antibody different from that used in the ELISA and then assayed these depleted samples. Following this strategy, we were able to specifically and almost completely remove immunoreactive AREG from the FF (Supplementary data, Fig. S1).
Figure 2.
(A) Distribution of AREG protein levels within individual human pre-ovulatory follicles. Individual follicular aspirates were collected 36 h after hCG, and AREG levels determined by ELISA. Each patient contributed a single FF specimen for the analysis. EREG was assayed by ELISA in 20 individual samples and was not detectable in 19 of these, and only present at a low level in one sample. BTC was not detectable in either individual or pooled fluid samples. (B) Follicular AREG is positively correlated with follicular hCG levels. Within individual follicles, AREG and hCG levels were determined and the results plotted. Follicular AREG and follicular hCG levels are positively correlated (P = 0.016).
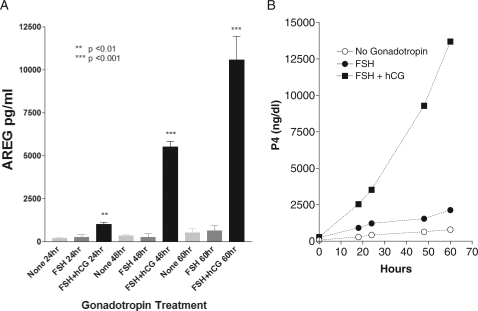
In the five patients available where FF was obtained prior to hCG administration, AREG was undetectable. A significant correlation between hCG concentration in the FF and AREG accumulation was established by comparing AREG and hCG levels within individual follicles P= 0.016 (Fig. 2B). The stringent dependence of AREG accumulation on gonadotrophin stimulation was further assessed in an in vitro system. Granulosa cells retrieved from mature-sized follicles accumulated AREG in the culture medium only when exposed to hCG (Fig. 3A). The FSH alone did not have a detectable effect in these luteinized granulosa cells. As expected, hCG increased progesterone secretion in these primary granulosa cell cultures when compared with either the FSH or the control (Fig. 3B).
Figure 3.
(A, B) AREG production and secretion is specifically mediated by LH signaling. Primary cell cultures were established from luteinized human granulosa cells obtained at the time of OR. Cells were then stimulated by either recombinant human FSH, hCG or vehicle only for up to 60 h. Cultured medium was assayed for AREG (Fig. 3A) or progesterone (Fig. 3B). Results for AREG production are mean ± SEM values from three different primary cell cultures taken from different patients. Statistical significance is in comparison with the no gonadotrophin stimulation at 24 h. Progesterone results for the primary granulosa cell culture are from an individual patient.
To define whether AREG accumulation is confined to the follicle, serum AREG levels were measured in five patients on the day prior to hCG administration and 36 h after hCG. Serum AREG levels were three orders of magnitude lower than in the FF and showed no significant change after hCG administration (pre-hCG mean 11 pg/ml SD 2.9; post-hCG mean 13 pg/ml SD 4.3).
A human EREG ELISA was developed with a sensitivity of ∼200 pg/ml and employed to assay 20 individual FF samples. Significant EREG accumulation was detected in only a single sample at a level of ∼400 pg/ml. A commercially available ELISA for BTC with a sensitivity of 15 pg/ml was used to assess four pooled samples of FF with known high levels of AREG, but no BTC was detected (data not shown). These data demonstrate that AREG is the most abundant EGF-like growth factor accumulating in the FF of the ovulatory follicle.
AREG in human FF is biologically active
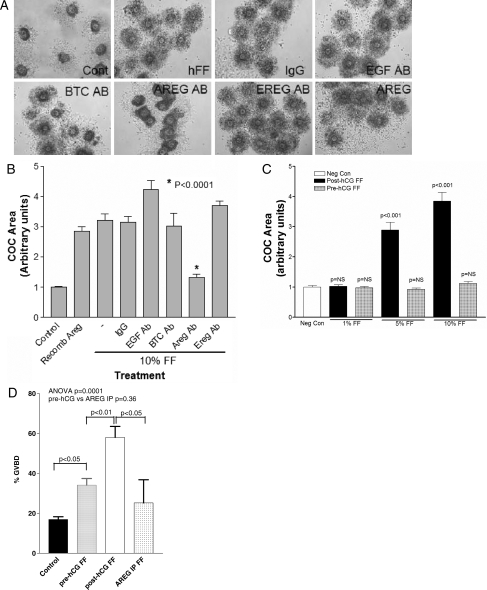
The biological activity of AREG derived from FF was assessed in three independent functional assays: COC expansion assay, an oocyte maturation assay and a conventional and widely used mitogenic assay for EGF-like growth factors. Figure 4C documents that human FF obtained after the hCG trigger, but not before hCG, causes CC expansion. Immunodepletion of AREG, but not EGF or other EGF-like growth factors, blocks the ability of post-hCG FF to cause this expansion (Fig. 4A and B). In the same vein, incubation of mouse COCs with human FF induces oocyte maturation (Fig. 4D). This stimulation was maximal with FF obtained after hCG trigger and was greatly reduced after immunodepletion of AREG. These results confirm the presence of biologically active AREG in human FF and suggest that AREG is a physiological mediator of oocyte maturation and CC expansion.
Figure 4.
(A) Biologically active AREG in human FF causes CC expansion. Mouse COCs were incubated with 10% human FF (post-hCG) which was immunodepleted (AB) of various EGF-like growth factors. AREG depletion specifically blocks normal CC expansion in this assay. Recombinant AREG (rAREG) is included as a control. Magnification: ×10. (B) Biologically active AREG in human FF causes CC expansion. A histogram showing the mean ± SEM of nine or more COCs analyzed in three separate experiments. Statistical significance determined relative to the mock immunoprecipitated 10% FF. (C) Human FF sampled before hCG administration (pre-hCG FF) does not promote COC expansion. (D) AREG in human FF induces oocyte nuclear maturation. Mouse COCs were incubated with 10% human FF (post-hCG) and assessed for nuclear maturation by light microscopy. Results are mean ± SEM of 15 or more COCs analyzed in three separate experiments. AREG IP, AREG removed by immunoprecipitation.
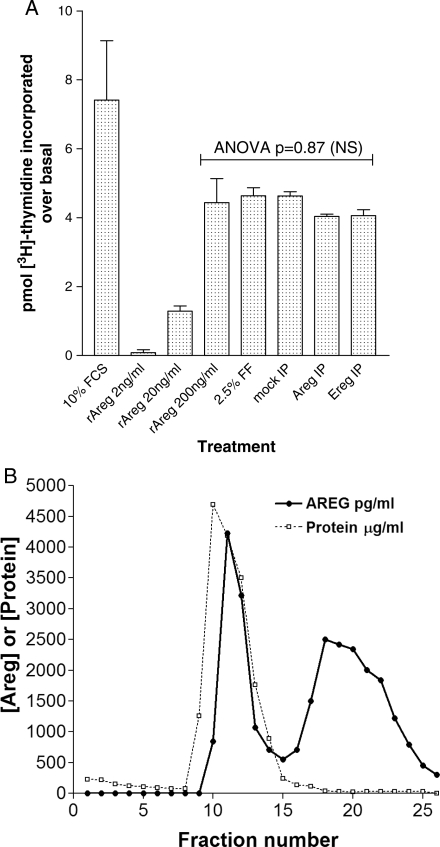
In a mitogenic assay, recombinant AREG caused the replication of 3T3 cells to the same extent as serum. Unfractionated FF was a potent mitogen in this particular assay. However, depletion of AREG did not diminish the mitogenic activity of this biological fluid. (Fig. 5A).
Figure 5.
(A) Mitogenic activity of ovarian FF is not abolished by AREG immunodepletion. [3H] Thymidine incorporation using murine Balb 3T3 cells stimulated with either fetal calf serum (FCS), various FF specimens (post-hCG) or rAREG. Results are expressed as pmol [3H] Thymidine over unstimulated controls. All samples were assayed in duplicate, and all values are mean ± SEM, with minimum n = 3. IP, immunoprecipitation. (B) Two major forms of AREG are distinguishable by ion exchange chromatography. Pooled human FF obtained 36 h after hCG was fractionated under the conditions described in Materials and Methods. Fractions were assayed for total protein by OD280 and BCA, and AREG levels by ELISA.
Multiple forms of AREG are identified by ion exchange chromatography
We next determined the physicochemical properties of AREG present in human FF compared with those of the recombinant protein. Utilizing mono-Q anion exchange chromatography, post-hCG human FF was fractionated and assayed for AREG and total protein. The column elution profile revealed two distinguishable forms of AREG, as shown in Fig. 5B. Recombinant AREG expressed in Escherichia coli elutes with a profile closer to the first peak than the second peak (data not shown). Presumably these forms in FF may represent either differently processed polypeptides or species of free and bound AREG. The fractions were then analyzed in the COC expansion assay, with the first AREG peak showing some activity for CC expansion but the second peak fractions caused oocyte degeneration, possibly related to the higher salt concentrations in the second peak fractions (data not shown). When the fractions from the anion exchange column were tested for mitogenic activity, no clear correlation with the AREG immunoreactivity could be established.
AREG levels as a predictor of oocyte quality
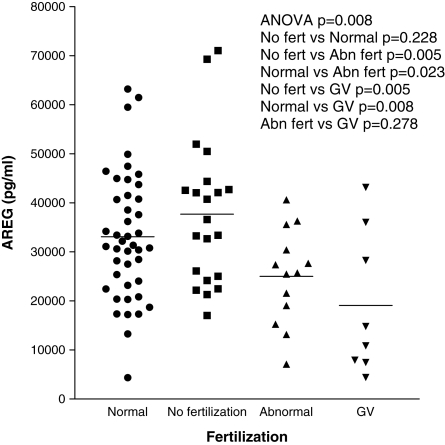
Using individual follicle aspirates, we correlated levels of AREG with clinically relevant parameters. We examined AREG levels in follicles yielding oocytes that did not fertilize, fertilized normally or fertilized abnormally with ICSI, as well as follicles yielding immature oocytes. Follicles producing immature GV oocytes have lower AREG levels than follicles producing nuclear mature metaphase II (MII) oocytes (P = 0.0071, mean 19 100 ± 5197 versus 33 000 ± 1533 pg/ml). Figure 6 shows that follicles yielding GV oocytes or abnormally fertilized oocytes (defined as any fertilization other than two pronuclei) have significantly lower levels of AREG than follicles yielding either normal or unfertilized oocytes. A similar analysis was performed on oocytes fertilized by conventional IVF; however, this showed no significant difference in AREG among the various fertilization groups (Supplementary data, Fig. S2). Correlations of FF AREG with other clinical parameters relevant to fertility treatment were assessed. There was no significant correlation between AREG and female age or follicle volume (Supplementary data, Figs S3 and S4).
Figure 6.
Levels of AREG in human ovarian follicles yielding normal oocytes or oocytes with compromised developmental potential. Human FF samples were assayed as described in the Materials and Methods. Each point represents a single FF sample with the bars showing the mean. Only a single FF sample was used from a given patient. Where possible, age-matched samples were used, with no significant differences in ages among samples in each of the groups. Data were stratified according to oocyte fertilization after ICSI, as assessed by an embryologist. Abnormal (Abn) embryos are defined as anything other than fertilization of two pronuclei. The GV group is a subgroup where individual follicles produced an immature oocyte.
Discussion
With this study, we provide evidence that gonadotrophin stimulation of the human ovulatory follicle produces massive accumulation of the EGF-like growth factor AREG in FF. This conclusion is supported by the finding that no immunoreactive AREG is detected in FF prior to the LH surge, that LH/hCG stimulates AREG secretion in vitro, that bioactive AREG appeared in FF after hCG stimulation, and that a positive correlation is established between hCG levels and AREG concentration in the FF of the mature-sized follicle. Given the biological effects of AREG and the presence of its cognate receptors, we propose that AREG is a critical signal for the gonadotrophin-dependent stimulation of cumulus expansion and oocyte maturation in humans. Although a wide range of AREG concentrations were measured in FF, follicles yielding an immature oocyte or an oocyte supporting aberrant embryo development showed significantly lower levels of AREG. Although these correlations need to be further refined taking into consideration the different AREG forms, they establish a clear link between the accumulation of this growth factor and oocyte quality.
In vitro data from human granulosa cell cultures as well as a recent study of IVF patients have shown that AREG is secreted by granulosa cells (Freimann et al., 2004; Negishi et al., 2007; Inoue et al., 2009). Our data confirm and extend these findings. Of note, a prior publication had noted a negative correlation of follicular AREG and follicular hCG which was only apparent in a small subset of patients (Inoue et al., 2009). The biological rationale of dividing patients in this manner and the use of pooled FF is likely to confound results, since our analysis of multiple individual aspirates from the same patient shows that the concentration of AREG in different follicles varies within the same patient (data not shown). This is the likely explanation for why we find a positive association of AREG and hCG which is consistent with both murine and in vitro human cultured granulosa cell data (Freimann et al., 2004; Park et al., 2004; Negishi et al., 2007).
Furthermore, we show that 36 h after hCG administration, in both CC and mGCs the predominant EGF-like growth factor mRNA expressed is AREG, with much lower levels of EREG and BTC mRNAs. This is consistent with our protein data showing no significant levels of EREG or BTC in FF. This is in contrast to murine studies, where up-regulation of all three EGF-like growth factors was seen after LH administration (Park et al., 2004) which could either reflect species-specific differences, such as differential LH receptor signaling, or be related to the sampling timing, since we do not have available FF at different times after hCG administration. One possibility is that EREG or BTC is expressed earlier than 36 h after hCG. Later expression is not likely to be biologically relevant for IVF since a substantial portion of IVF cases have removal of the CCs 4–6 h after OR in order to perform ICSI. Given that AREG is present in FF after hCG administration, we tested whether AREG was detectable in serum and if this could be used as a correlate for FF AREG levels, but we were unable to find any measurable clinical differences in serum level. This result is not necessarily surprising since the production of AREG would only occur for 36 h and it may not have had time to diffuse into the serum, or it could reflect degradation.
The AREG accumulating in FF is biologically active, because it supports both oocyte maturation and cumulus expansion. Previous work has identified multiple pathways and ligands which are important for CC expansion (Downs, 1989; Buccione et al., 1990; Chen et al., 1993; Dragovic et al., 2005; Pangas and Matzuk, 2005; Diaz et al., 2006; Fan et al., 2008; Kawashima et al., 2008; Yeo et al., 2009). Both in vitro and in vivo studies suggest that multiple signals are involved in CC expansion (Russell and Salustri, 2006). It has been previously reported that human FF causes murine CC expansion (Das et al., 1992) and that recombinant AREG also produces a similar effect (Park et al., 2004). Our study suggests that AREG is physiologically a critical ligand for this process. Under our experimental conditions, recombinant AREG alone is sufficient to cause COC expansion, although at a concentration significantly higher than that detected in FF (100 nM versus ∼5 nM, respectively). There are two possible explanations for this discrepancy. Other factors in the fluid may be contributing either directly or indirectly to AREG's ability to cause COC expansion; indeed oocyte-derived growth factors, such as GDF-9, function as enabling factors for cumulus expansion and GDF-9 is detected in human ovarian follicles (Aaltonen et al., 1999). Alternatively, the potencies of the native and recombinant forms of AREG are different. The recombinant protein is expressed in E. coli and therefore may have aberrant post-translational modifications.
The findings of AREG's importance in the physiologically critical processes of oocyte maturation and CC expansion provide a mechanistic explanation for the current use of EGF to improve in vitro maturation (IVM) conditions. On the basis of findings in several animal models, previous work has examined the effects of EGF on human IVM conditions and shown that addition of this growth factor can improve IVM outcomes (Das et al., 1991; Gomez et al., 1993). The addition of physiological concentrations of EGF to IVM culture media has been shown to promote both nuclear and cytoplasmic maturation of human GV oocytes, with the finding that the improvement in nuclear maturation was marked for denuded oocytes (an 2-fold increase in rates of MII formation) versus a small non-significant increase in MII formation for cumulus-enclosed oocytes (Goud et al., 1998). Additional work in rhesus monkeys has shown that AG1478 (a chemical EGF receptor inhibitor) decreased oocyte maturation, cumulus expansion and blastocyst formation in vitro, highlighting the critical importance of EGFR activation for proper oocyte maturation in vitro (Nyholt de Prada et al., 2009). In this model, additional EGF did not improve conditions, leading the authors to conclude that EGF-like peptides from CCs may already be providing maximal EGF receptor stimulation (Nyholt de Prada et al., 2009).
Previous studies have shown that AREG is synthesized as a 252 amino acid transmembrane precursor with multiple N-linked and O-linked glycosylation sites (Shoyab et al., 1988; Sanderson et al., 2006). Differential proteolytic cleavage is thought to generate two different AREG isoforms of slightly different molecular weights (78 and 84 amino acids). Our chromatography studies suggest the presence of either two isoforms of AREG, or a free and bound species of AREG in human FF. For technical reasons, we could not directly compare the biological activities of these two peaks. We did show in a mitogenic assay that FF mitogenic activity did not correlate with AREG immunoreactivity in the column fractions, and that FF depleted of AREG had mitogenic activity similar to native FF. This finding demonstrates that additional mitogens accumulate in the FF prior to the ovulatory process. Future work will focus on the potencies of these two different chromatographic species to determine whether they are structurally different isoforms or represent AREG complexed with an as yet undefined binding partner, and how this may differ in different cell populations.
Since our in vitro studies show that AREG is biologically active and up-regulated by hCG, we examined levels of AREG in individual follicular aspirates to determine if there was any correlation with certain patient characteristics or oocyte-related outcomes, such as fertilization and early embryo growth. We did not find a significant correlation between AREG and female age or follicle size. We did find that GV oocytes and abnormally fertilized oocytes with ICSI had significantly lower AREG levels than either unfertilized or normal, two pronuclei, fertilized oocytes. With ICSI, it is assumed that an abnormal number of pronuclei is caused by an oocyte defect, through mechanisms such as failed polar body extrusion or failed coordination of nuclear and cytoplasmic divisions. This may represent incorrect cytoplasmic maturation, since with ICSI only MII oocytes are used, controlling nuclear maturation. Similarly, GV oocytes retrieved after hCG are presumed to have an abnormality which prevented proper oocyte maturation. A similar analysis was performed on conventional insemination samples but did not show any significant differences among the fertilization groups. We had hypothesized that given the importance of AREG for COC expansion, a subset of unfertilized oocytes from conventional IVF would result from poor cumulus expansion, and that this sample would have lower AREG levels. The fact that we did not see this could be explained by a threshold effect where AREG was already at high enough levels in the majority of the samples, or the limited sample size available and the inability to precisely assess the oocyte maturation stage with conventional IVF. Furthermore, certain physiological conditions with altered LH responsiveness or altered granulosa cell physiology, such as polycystic ovarian syndrome, may have abnormal AREG production leading to suboptimal results from ovarian stimulation during fertility treatment. This is an important clinical question, which warrants further study.
Taken together, our findings strongly support a critical role for AREG during the peri-ovulatory period as an intrafollicular signal. However, the function of this growth factor may extend beyond oocyte maturation and cumulus expansion. Together with the demonstration that additional mitogens are present in the FF, the high concentration of AREG indicates that factors with high levels of mitogenic activity are released in the peritoneal cavity during each ovulation. An additional, extrafollicular physiological function for these mitogens may be facilitation of the healing process of the surface of the ovary after ovulation. If this is correct, a burst of cell replication is induced by AREG and other mitogens during each ovulatory event. In addition to healing of the ovarian epithelium, these mitogens may promote the selection and expansion of ovarian epithelial cells carrying oncogenic mutations. The levels of AREG in FF are at least 100-fold higher than the levels reported in the serum of cancer patients with tumors expressing AREG, or in conditioned media from human cancer cell lines known to express AREG (Ishikawa et al., 2005; Yotsumoto et al., 2008). Thus, the massive peri-ovulatory AREG accumulation may establish a functional link between repetitive ovulations and increased risk of ovarian epithelial carcinomas (Purdie et al., 2003; Ben-Ami et al., 2006).
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Funding
The work reported was supported by NIH R01 HD052909 to MC.
Supplementary Material
Acknowledgements
We are grateful to Dr Luigi Devoto and Dr C. Villaroel Quintana for sharing follicular fluid from a patient undergoing tubal ligation.
Conflict of interest: none declared.
References
- Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, Louhio H, Tuuri T, Sjoberg J, Butzow R, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84:2744–2750. doi: 10.1210/jcem.84.8.5921. doi:10.1210/jc.84.8.2744. [DOI] [PubMed] [Google Scholar]
- Adam RM, Borer JG, Williams J, Eastham JA, Loughlin KR, Freeman MR. Amphiregulin is coordinately expressed with heparin-binding epidermal growth factor-like growth factor in the interstitial smooth muscle of the human prostate. Endocrinology. 1999;140:5866–5875. doi: 10.1210/endo.140.12.7221. doi:10.1210/en.140.12.5866. [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. doi:10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Ben-Ami I, Freimann S, Armon L, Dantes A, Ron-El R, Amsterdam A. Novel function of ovarian growth factors: combined studies by DNA microarray, biochemical and physiological approaches. Mol Hum Reprod. 2006;12:413–419. doi: 10.1093/molehr/gal045. doi:10.1093/molehr/gal045. [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol. 1990;138:16–25. doi: 10.1016/0012-1606(90)90172-f. doi:10.1016/0012-1606(90)90172-F. [DOI] [PubMed] [Google Scholar]
- Chandrasekher YA, Hutchison JS, Zelinski-Wooten MB, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in primate follicles using recombinant and native human luteinizing hormone to mimic the midcycle gonadotropin surge. J Clin Endocrinol Metab. 1994;79:298–306. doi: 10.1210/jcem.79.1.8027245. doi:10.1210/jc.79.1.298. [DOI] [PubMed] [Google Scholar]
- Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev. 1993;34:87–93. doi: 10.1002/mrd.1080340114. doi:10.1002/mrd.1080340114. [DOI] [PubMed] [Google Scholar]
- Das K, Stout LE, Hensleigh HC, Tagatz GE, Phipps WR, Leung BS. Direct positive effect of epidermal growth factor on the cytoplasmic maturation of mouse and human oocytes. Fertil Steril. 1991;55:1000–1004. doi: 10.1016/s0015-0282(16)54313-1. [DOI] [PubMed] [Google Scholar]
- Das K, Phipps WR, Hensleigh HC, Tagatz GE. Epidermal growth factor in human follicular fluid stimulates mouse oocyte maturation in vitro. Fertil Steril. 1992;57:895–901. doi: 10.1016/s0015-0282(16)54977-2. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. doi:10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Downs SM. Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod. 1989;41:371–379. doi: 10.1095/biolreprod41.2.371. doi:10.1095/biolreprod41.2.371. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology. 2005;146:2798–2806. doi: 10.1210/en.2005-0098. doi:10.1210/en.2005-0098. [DOI] [PubMed] [Google Scholar]
- Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. doi: 10.1242/dev.020560. doi:10.1242/dev.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. doi:10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324:829–834. doi: 10.1016/j.bbrc.2004.09.129. doi:10.1016/j.bbrc.2004.09.129. [DOI] [PubMed] [Google Scholar]
- Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod. 2007;22:1247–1252. doi: 10.1093/humrep/del519. doi:10.1093/humrep/del519. [DOI] [PubMed] [Google Scholar]
- Gomez E, de los Santos MJ, Ruiz A, Tarin JJ, Remohi J, Pellicer A. Effects of epidermal growth factor in the final stages of nuclear and cytoplasmic oocyte maturation in humans. Hum Reprod. 1993;8:691–694. doi: 10.1093/oxfordjournals.humrep.a138121. [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, Dhont M. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–1644. doi: 10.1093/humrep/13.6.1638. doi:10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46. doi: 10.1016/s0303-7207(01)00469-5. doi:10.1016/S0303-7207(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. doi:10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Miyamoto S, Fukami T, Shirota K, Yotsumoto F, Kawarabayashi T. Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2009;91:1035–1041. doi: 10.1016/j.fertnstert.2008.01.014. doi:10.1016/j.fertnstert.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Daigo Y, Takano A, Taniwaki M, Kato T, Hayama S, Murakami H, Takeshima Y, Inai K, Nishimura H, et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005;65:9176–9184. doi: 10.1158/0008-5472.CAN-05-1556. doi:10.1158/0008-5472.CAN-05-1556. [DOI] [PubMed] [Google Scholar]
- Kawashima I, Okazaki T, Noma N, Nishibori M, Yamashita Y, Shimada M. Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation-related genes that impact oocyte maturation in vivo and in vitro. Reproduction. 2008;136:9–21. doi: 10.1530/REP-08-0074. doi:10.1530/REP-08-0074. [DOI] [PubMed] [Google Scholar]
- Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol. 2004;24:8907–8916. doi: 10.1128/MCB.24.20.8907-8916.2004. doi:10.1128/MCB.24.20.8907-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Jimenez-Krassel F, Ireland JJ, Smith GW. Gene expression profiling of bovine preovulatory follicles: gonadotropin surge and prostanoid-dependent up-regulation of genes potentially linked to the ovulatory process. Reproduction. 2009;137:297–307. doi: 10.1530/REP-08-0308. doi:10.1530/REP-08-0308. [DOI] [PubMed] [Google Scholar]
- Negishi H, Ikeda C, Nagai Y, Satoh A, Kumasako Y, Makinoda S, Ustunomiya T. Regulation of amphiregulin, EGFR-like factor expression by hCG in cultured human granulosa cells. Acta Obstet Gynecol Scand. 2007;86:706–710. doi: 10.1080/00016340701314959. doi:10.1080/00016340701314959. [DOI] [PubMed] [Google Scholar]
- Nyholt de Prada JK, Lee YS, Latham KE, Chaffin CL, VandeVoort CA. Role for cumulus cell-produced EGF-like ligands during primate oocyte maturation in vitro. Am J Physiol Endocrinol Metab. 2009;296:E1049–E1058. doi: 10.1152/ajpendo.90930.2008. doi:10.1152/ajpendo.90930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Matzuk MM. The art and artifact of GDF9 activity: cumulus expansion and the cumulus expansion-enabling factor. Biol Reprod. 2005;73:582–585. doi: 10.1095/biolreprod.105.042127. doi:10.1095/biolreprod.105.042127. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. doi:10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104:228–232. doi: 10.1002/ijc.10927. doi:10.1002/ijc.10927. [DOI] [PubMed] [Google Scholar]
- Reeka N, Berg FD, Brucker C. Presence of transforming growth factor alpha and epidermal growth factor in human ovarian tissue and follicular fluid. Hum Reprod. 1998;13:2199–2205. doi: 10.1093/humrep/13.8.2199. doi:10.1093/humrep/13.8.2199. [DOI] [PubMed] [Google Scholar]
- Rosen MP, Zamah AM, Shen S, Dobson AT, McCulloch CE, Rinaudo PF, Lamb JD, Cedars MI. The effect of follicular fluid hormones on oocyte recovery after ovarian stimulation: FSH level predicts oocyte recovery. Reprod Biol Endocrinol. 2009;7:35. doi: 10.1186/1477-7827-7-35. doi:10.1186/1477-7827-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24:217–227. doi: 10.1055/s-2006-948551. doi:10.1055/s-2006-948551. [DOI] [PubMed] [Google Scholar]
- Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors. 2006;24:121–136. doi: 10.1080/08977190600634373. doi:10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]
- Shing Y, Christofori G, Hanahan D, Ono Y, Sasada R, Igarashi K, Folkman J. Betacellulin: a mitogen from pancreatic beta cell tumors. Science. 1993;259:1604–1607. doi: 10.1126/science.8456283. doi:10.1126/science.8456283. [DOI] [PubMed] [Google Scholar]
- Shoyab M, McDonald VL, Bradley JG, Todaro GJ. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci USA. 1988;85:6528–6532. doi: 10.1073/pnas.85.17.6528. doi:10.1073/pnas.85.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/endo.143.6.8845. doi:10.1210/en.143.6.2221. [DOI] [PubMed] [Google Scholar]
- Sullivan MW, Stewart-Akers A, Krasnow JS, Berga SL, Zeleznik AJ. Ovarian responses in women to recombinant follicle-stimulating hormone and luteinizing hormone (LH): a role for LH in the final stages of follicular maturation. J Clin Endocrinol Metab. 1999;84:228–232. doi: 10.1210/jcem.84.1.5389. doi:10.1210/jc.84.1.228. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Komurasaki T, Uchida D, Takayama Y, Isobe T, Okuyama T, Hanada K. Epiregulin. A novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J Biol Chem. 1995;270:7495–7500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- Westergaard LG, Andersen CY. Epidermal growth factor (EGF) in human preovulatory follicles. Hum Reprod. 1989;4:257–260. doi: 10.1093/oxfordjournals.humrep.a136883. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kawashima I, Yanai Y, Nishibori M, Richards JS, Shimada M. Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology. 2007;148:6164–6175. doi: 10.1210/en.2007-0195. doi:10.1210/en.2007-0195. [DOI] [PubMed] [Google Scholar]
- Yeo CX, Gilchrist RB, Lane M. Disruption of bi-directional oocyte-cumulus paracrine signaling during in vitro maturation reduces subsequent mouse oocyte developmental competence. Biol Reprod. 2009;80:1072–1080. doi: 10.1095/biolreprod.108.073908. doi:10.1095/biolreprod.108.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, Hachisuga T, Sonoda K, Kawarabayashi T, Mekada E, Miyamoto S. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–561. doi: 10.1016/j.bbrc.2007.11.015. doi:10.1016/j.bbrc.2007.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.