Figure 1.
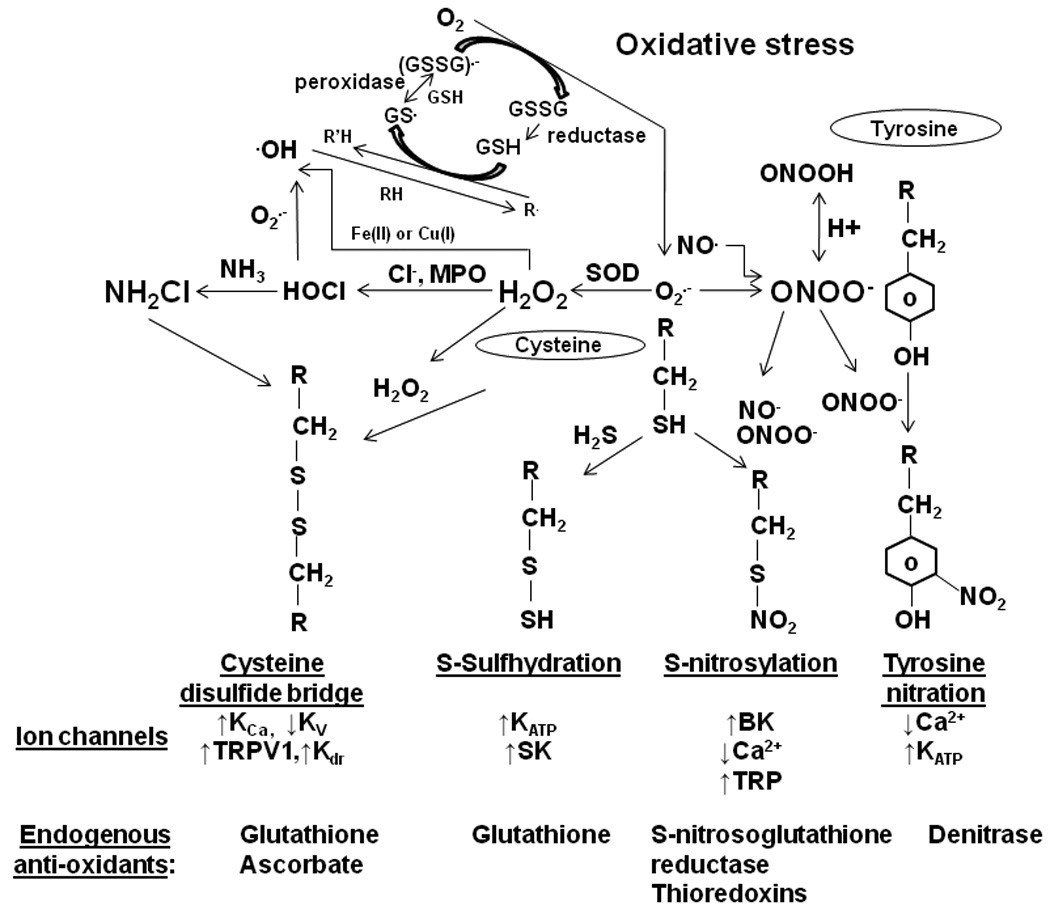
Schematic presentation of pathways associated with post-translational modification of amino acid residues of ion channels. The central components are the presence of reactive superoxide, nitric oxide and hydrogen sulfide. The dismutation of superoxide leads to the production of hydrogen peroxide (H2O2) that oxidizes cysteine residues resulting in disulfide bonds between two approximating thiols groups. Cysteine residues can also be modified by monochloramine (NH2Cl) that is produced from interaction of H2O2 with ammonia (NH3). Nitric oxide can directly s-nitrosylate the sulfhydryl group of the cysteine residues or combine with superoxide to generate peroxynitrite (ONOO−). Peroxynitrite interacts with tyrosine residues resulting in 3-nitrotyrosine that prevents tyrosine phosphorylation. Hydrogen sulfide interacts with cysteine residues to produce hydropersulfide. Ion channels are directly modified by phosphorylation and post-translational modification can also be altered/prevented by endogenous anti-oxidants
