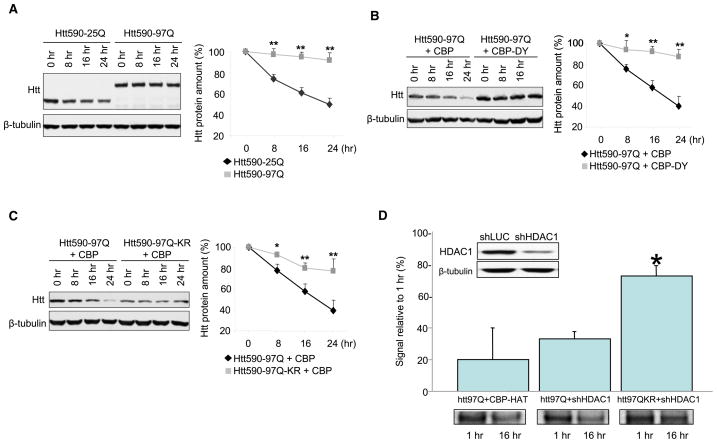
Figure 4. Acetylation of K444 Promotes Clearance of Mutant Htt.
Neuro2a cells were transfected with Htt and CBP-HAT, treated with cycloheximide (CHX), and harvested at the indicated time points. Western blots were analyzed by densitometry and values normalized to the amount of Htt at the time of cycloheximide treatment (100%). Values represent means of at least four independent experiments + SEM; *p < 0.05; **p < 0.01 compared to Htt590-25Q (A), Htt590-97Q + CBP-HAT (B), and Htt590-97Q + CBP-HAT (C), respectively.
(A) Mutant Htt590-97Q has a longer half-life than wild-type Htt590-25Q.
(B) Cotransfection of CBP-HAT, but not the HAT-deficient CBP-HAT-DY, resulted in increased clearance of mutant Htt.
(C) Acetylation-induced clearance of mutant Htt depends on K444. Coexpression of CBP-HAT led to increased clearance of Htt590-97Q, whereas mutation of Htt K444 to R prevented this effect of CBP-HAT.
(D) Enhanced clearance of Htt590-97Q by HDAC1 knockdown requires residue K444. Efficient knockdown of HDAC1 was achieved 3 days after transfection of pSUPER-HDAC1 compared to transfection of pSUPER-luciferase in Neuro2a cells. β-tubulin was used as loading control (inset). Levels of Htt590-97Q were monitored by radiolabeled pulse-chase. Knockdown of HDAC1 led to significant clearance of Htt590-97Q (32.6% ± 4.9%), comparable to levels achieved with coexpression of CBP-HAT (20.0% ± 16.9%). Mutagenesis of K444 to R (K444R) led to a significant decrease of this clearance (73.0% ± 8.5%). Densitometry measurements are expressed as % of signal after a 1 hr chase. Three independent experiments were performed and are represented as mean + SEM. *p < 0.001 compared to Htt97Q+shHDAC1.