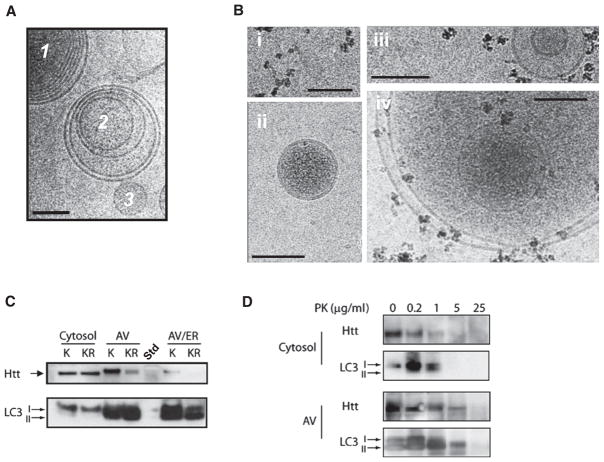
Figure 6. Acetylated Mutant Htt Is Trafficked to Autophagosomes.
(A) Purification of autophagosomes (AV). Neuro2A cells were fractionated and AV, lysosomes, and endoplasmic reticulum (ER) isolated using two distinct protocols, as described in Experimental Procedures. Cryo-EM revealed fractions enriched in multivesicular bodies that are morphologically consistent with AV (1, 2) and unilamellar structures like transport vesicles (3).
(B) To determine whether LC3 was associated with membrane structures, AV fractions were incubated with an antibody against LC3 followed by iron-rich magnetic beads conjugated to Protein A. (i) Beads imaged alone. (ii) Unilamellar transport vesicles were not labeled by beads. (iii and iv) Multilamellar autophagosomal-like particles are labeled by beads.
(C) Preferential trafficking of acetylated Htt to autophagosomes. Cells were fractionated and AV isolated, run on SDS-PAGE, and probed with anti-Htt antibody or LC3 antibody. Htt590-97Q was preferentially detected in the AV-enriched fractions compared to Htt590-97QKR. The cytosolic fractions predominantly contain the unbound form of LC3 (LC3-I), while the AV-enriched fractions contain the membrane-bound form of LC3 (LC3-II). Fraction “AV/ER” differs from “AV” as it is also enriched for ER markers such as calnexin (not shown). Std, protein standard.
(D) Mutant Htt is detected within the AV membrane fraction. Protease protection assay was performed with 20 μg of protein from a cytosol fraction or AV fraction collected from (C), subjected to increasing amounts of proteinase K (PK) and examined by SDS-PAGE. In the cytosolic fraction, both Htt590-97Q and the LC3-I were degraded after exposure to 1 μg/ml PK. In the AV fraction, Htt590-97Q did not degrade until 25 μg/ml PK when the LC3-II was also degraded. Htt590-97Q was detected using MAB5492 (Chemicon) and LC3 with rabbit polyclonal antibody (Abcam). A representative image from three independent experiments is shown.