Abstract
The prevalence of obesity in industrialized societies has become markedly elevated. In contrast, model organism research shows that reducing caloric intake below ad libitum levels provides many health and longevity benefits. Despite these benefits, few people are willing and able to reduce caloric intake over prolonged periods. Prior research suggests that mannooligosaccharide (MOS or Mannan) supplementation can increase lifespan of some livestock and in rodents can reduce visceral fat without reducing caloric intake. Hence, we tested the effect of MOS supplementation as a possible calorie restriction mimetic in mice. C57Bl/6J male mice were fed a high-fat “western” type diet with or without 1% MOS (by weight) supplementation (n=24/group) from 8 to 20 weeks of age. Animals were housed individually and provided 95% of ad libitum food intake throughout the study. Body weight was measured weekly and body composition (lean and fat mass) measured non-invasively every 3 weeks. Individual fat depot weights were acquired by dissection at study completion. Supplementation of a high-fat diet with 1% MOS tended to reduce total food intake (mean±s.d.; CON: 293.69±10.53g, MOS: 288.10±11.82g; p=0.09) during the study. Moreover, MOS supplementation had no significant effect on final body weight (CON: 25.21±2.31g, MOS: 25.28±1.49g; p=0.91), total fat (CON: 4.72±0.90g, MOS: 4.82±0.83g; p=0.69) or visceral fat (CON: 1.048±0.276g, MOS: 1.004±0.247g; p=0.57). Contrary to previous research, MOS supplementation had no discernable effect on body weight gain or composition during this 12-week study, challenging the potential use of MOS as a calorie restriction mimetic or body composition enhancer.
Keywords: Fiber, Mannan, Oligosaccharide, MOS, Fat, Visceral Fat, Adipose Tissue, Obesity, Caloric Restriction
Introduction
The prevalence of obesity in industrialized nations has risen. Obesity contributes to many health problems and impairs longevity (1, 2). Coincident with research aimed at understanding the genetic and molecular basis of this growing epidemic, nutritionists are seeking alternative methods to reduce adiposity as current obesity treatments do not appear to work for all individuals and, with the exception of surgery, are only of modest efficacy, and often associated with unacceptable side-effects (3, 4).
Calorie restriction (CR) has been shown to provide many health benefits since its description over 70 years ago (5, 6). CR remains the most reproducible intervention to increase lifespan in laboratory organisms, while delaying or preventing many of the age-related diseases that develop in the ad libitum fed state (7). Although the exact mechanism of CR’s benefit remains unclear, reduced adiposity, particularly visceral adiposity, may play a role in aging and CR-induced extension of lifespan (8, 9). Observational and randomized controlled trials in humans have provided promising preliminary results suggesting a CR dietary intervention might also have beneficial effects in humans were it implementable (10, 11). Yet a widely noted limitation of CR as a potential intervention in humans is the low likelihood that humans would accept and be able to adhere to a diet that requires a significant reduction in caloric intake throughout life. Many, if not most, of the rodent studies of CR implement a 30% or greater reduction of calories (12, 13). Less severe restriction is suggested to provide benefits proportional to the amount of restriction (14). A substance or intervention that allowed normal food intake while reproducing some of the benefits associated with CR – referred to as a CR ‘mimetic’ (CRM) – would therefore be of great utility (15, 16).
Recent studies suggest mannanoligosaccharides (MOS) may be such a compound. MOS supplementation reduced mortality rate in livestock studies (17). Laboratory studies have reported reduced fat absorption, resulting in reduced weight and visceral fat while maintaining normal food intake in rodents and humans (18–22). Fiber-like compounds have been observed to produce a variety of beneficial effects and the ability to add them to existing dietary components makes them attractive candidates for general supplementation, particularly if these mimic the effects of reduced calorie intake.
Mannan is a mannose-based polysaccharide found in many plants – coffee, certain beans, etc. – as well as in yeast cell walls (23). Glucomannans, isolated from konjac mannan, have been used in Asia for centuries as a thickening agent (24). Because mammals do not possess the enzymes necessary to cleave the β-1,4 linked sugar residues of the mannan backbone, these polymers are believed to pass through the upper gastro-intestinal (GI) tract undigested and encounter some amount of hydrolysis and uptake by bacteria in the colon, where mannan and MOS are proposed to selectively enhance the bifidobacteria species (25–30). Glucomannan supplementation promotes weight loss in overweight and obese individuals, purportedly through the fiber-fill effect and reduced fat uptake of through the GI tract (31). Non-digestible oligosaccharides and soluble fibers are likewise reported to alter the uptake of various minerals from the intestine, promoting bone health and maintenance in animal models (32–34).
The purpose of this study was to evaluate the effect of a purified MOS supplemented diet on body weight and composition in a mouse model, to see if we could replicate results showing decreased visceral fat as a result of MOS consumption, despite similar food intake, thereby supporting the potential of MOS as a CR mimetic.
Methods
Animals
Six week-old male C57Bl/6J mice were acquired from The Jackson Laboratory. Mice were singly housed in polycarbonate, standard mouse cages with filter tops, containing ~200 grams of sterilized Beta ChipR bedding (NEPCO, Warrensburg, NY) and an iso-BLOX Enrichment Square (#6060 – Harlan/Teklad, Madison, WI). Mice were acclimated to the University of Alabama at Birmingham (UAB) animal facilities (specific pathogen free conditions with sentinel monitoring) for one week after arrival. Mice were maintained at 22±2°C with a 12:12 light:dark cycle throughout the study. Body weights were measured weekly to the nearest 0.01 gram until study completion.
Diet
At seven weeks of age, all mice we given ad libitum (AL) access to the control high-fat (western type) diet manufactured by Bio-serv, Inc. (Frenchtown, NJ, USA) (for diet composition see Table 1). At eight weeks of age, mice were divided into two groups and either continued with the high-fat western type diet (control – CON, n=24) or were alternatively placed on the high-fat diet supplemented with 1% MOS (#KL903 – 95% purity, Wuhan Kaili Medical & Sanitary Products, Co., LTD, Wuhan, China) by weight (MOS, n=24) (Table 1). Additional purity and chemical composition of the MOS is described in the online Supplementary Material (www.nature.com/obesity). One gram precision, dustless food pellets (BioServ) were divided to improve accessibility using the available feeding apparatus. In a 4-week pre-study experiment, food intake was not significantly different between the high-fat control and MOS supplemented diets (p=0.15, data not shown). Combined total daily intake per animal was used to determine the 95% food allotment (3.74g/mouse per day) for the 12-week study. Both the CON and MOS groups were fed daily at 1600 hrs (2 hrs before lights out) during the 12-week study (8–20 weeks of age). Food intake was monitored daily and any remaining amounts of the 95% allotment were recorded to the nearest 0.01 gram and discarded.
Table 1.
Diet Description – Control and MOS diet composition and macronutrient content with calculated relative macronutrient caloric values.
| Ingredients | Control (gm/kg) | 1% MOS (gm/kg) |
|---|---|---|
| Dextrates | 433.906 | 423.856 |
| Casein | 200.00 | 200.00 |
| Sucrose | 32.58 | 32.58 |
| Soybean Oil | 20.00 | 20.00 |
| Hydr. Vegetable Oil | 213.33 | 213.33 |
| Fiber (Cellulose BW 200) | 50.00 | 50.00 |
| Vitamin Mix | 10.00 | 10.00 |
| L-Cystine | 3.00 | 3.00 |
| Choline Bitartrate | 2.50 | 2.50 |
| t-BHQ | 0.014 | 0.014 |
| MOS | - | 10.50 |
| Flowing Agents | Added | Added |
| Calculated Values | % grams | % kcal | % grams | % kcal |
|---|---|---|---|---|
| Carbohydrate | 45.9 | 39.4 | 45.9 | 39.4 |
| Protein | 18.0 | 15.5 | 18.0 | 15.5 |
| Fat | 23.3 | 45.1 | 23.3 | 45.1 |
| Fiber | 5.0 | - | 5.0 | - |
| Ash | 2.8 | - | 2.8 | - |
| Moisture | <5.0 | - | <5.0 | - |
| Energy (kcal/gm) | 4.653 | 4.653 |
Body Composition Measures
In vivo body composition measures of lean and fat mass were determined by Quantitative Magnetic Resonance (QMR, EchoMRI 3-in-1, Echo Medical System, Houston, TX USA) at baseline (age 8 weeks) and every 3rd week thereafter (age 11, 14, 17 and 20 weeks). Dual-energy x-ray absorption (DXA, GE Lunar PIXImus, Lunar, Madison, WI) measures of bone mineral density (BMD – grams/cm2), bone mineral content (BMC – grams) and area (cm2) were also obtained for a subset of mice (12/group) at baseline (8 weeks of age) and the end of the study (20 weeks of age). BMD, BMC and area were analyzed using PIXImus software version 1.45. At the completion of the study, animal carcasses were dissected and individual fat pads (inguinal white adipose tissue – IWAT, dorsal subcutaneous – SWAT, gonadal – GWAT, retroperitoneal – RWAT, mesenteric – MWAT, and interscapular brown adipose tissue – IBAT) measured for wet weight to the nearest 0.001 grams. All procedures were performed in accordance with Institutional Animal Care and Use Committee at UAB.
Statistics
Data were analyzed with SAS 9.1 statistical software (SAS Institute, Cary, NC, USA). Food intake, body weight, fat mass, lean mass and % fat were analyzed with a group by time repeated measures analysis of variance (ANOVA). A post-hoc Bonferroni adjustment was used for multiple comparisons in body weight and food intake. Group differences between CON and MOS for total food intake, final body weight, final body composition, BMD, BMC, bone area and fat pad weights were determined by Student t-test. Results were considered significant when p≤0.05 (two-tailed).
Results
Food Intake and Body Weight
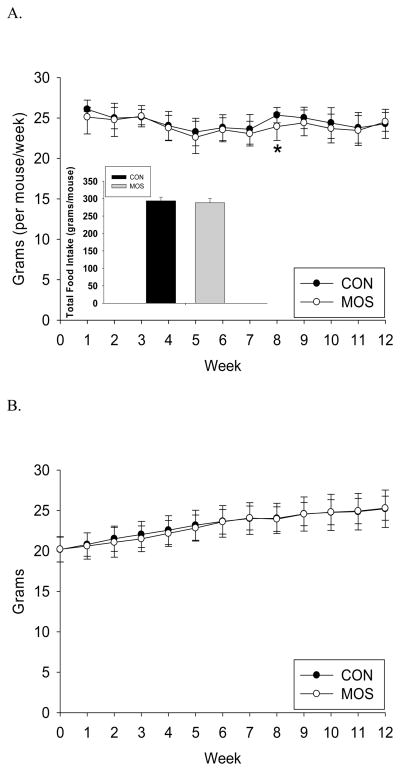
As seen in figure 1.A., total food intake (grams/mouse) was lower in the MOS group, but the difference was not statistically significant between groups (mean±s.d.: CON=293.69±10.53g, MOS=288.10±11.82g; p=0.09). Weekly food intake was significantly different between the groups at week 8 (p<0.01) after applying Bonferroni correction for multiple comparisons (Fig. 1.A.). Likewise, final body weight was not significantly different between the 2 groups (CON: 25.21±2.31g, MOS: 25.28±1.49g; p=0.91) or at any week during the study (Fig. 1.B.).
Figure 1.
Food intake and body weight. A) Weekly food intake (grams/mouse) by group (CON: n=24; MOS: n=24) during the 12 week study with inset showing total food intake (grams/mouse) by group (mean±s.d.). B) Body weight (grams/mouse) by group (CON: n=24; MOS: n=24) during the 12 week study. * denotes significance at p<0.01.
Body Composition
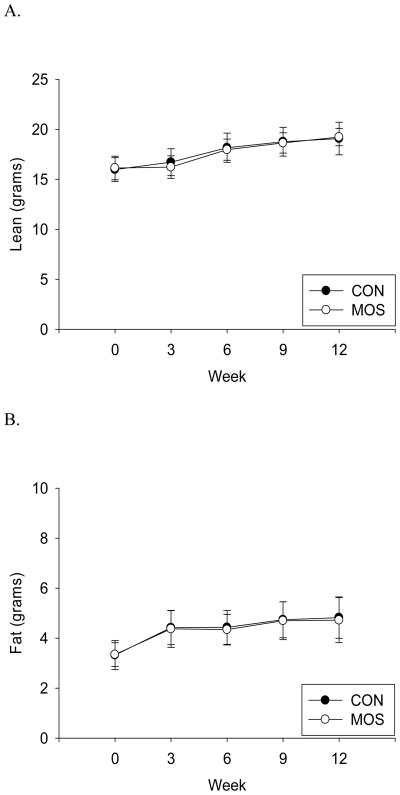
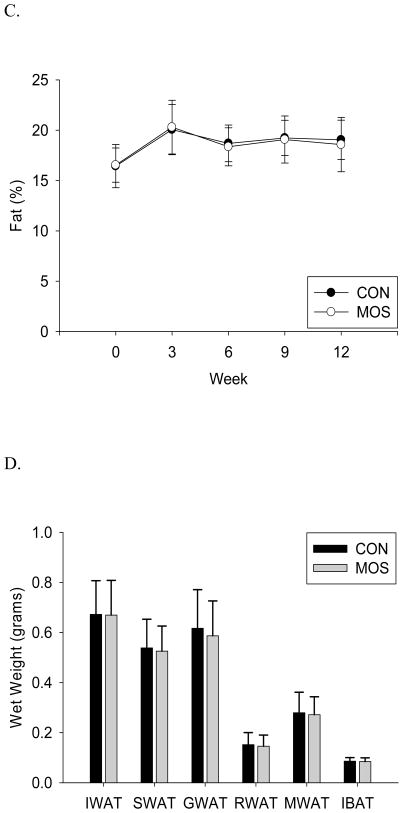
In vivo body composition analysis revealed that total body fat and lean mass were not significantly different between CON and MOS supplemented animals (Fig. 2.A.&B.). Similarly, calculated percentage fat was not significantly different between the 2 groups (Fig. 2.C.) There were no significant differences between CON and MOS groups for any dissected fat pad weights, visceral fat (GWAT+RWAT+MWAT: p=0.566) or total fat pad mass (p=0.667; Fig. 2.D.). Bone mineral density, bone mineral content and area were also not significantly different between the groups (CON vs. MOS (mean±s.d.), p-value: BMD=0.0431±0.0018 vs. 0.0439±0.0014, p=0.26; BMC=0.348±0.032 vs. 0.354±0.017, p=0.57; Area=8.10±0.52 vs. 8.08±0.20, p=0.90).
Figure 2.
Body composition and individual fat depot mass. A) Lean mass (in grams) (p-value: wk 3=0.17, wk 6=0.59, wk 9=0.73, wk 12=0.70) B) fat mass (in grams) (p-value: wk 3=0.81, wk 6=0.63, wk 9=0.85, wk 12=0.69) and C) percent fat (fat mass/body weight) by group (CON: n=24; MOS: n=24) measured by QMR every 3 weeks until study completion (mean±s.d.). D) Dissected wet weight (mean±s.d.) of fat depots (IWAT = inguinal white adipose tissue, SWAT = subcutaneous WAT, GWAT = gonadal WAT, RWAT = retroperitoneal WAT, MWAT = mesenteric WAT, IBAT = interscapular brown adipose tissue) by group (CON: n=24; MOS: n=24) (IWAT: p=0.945; SWAT: p=0.670; GWAT: p=0.483; RWAT: p=0.666; MWAT: p=0.722; IBAT: p=0.713).
Discussion
Although MOS supplementation has been reported to reduce body weight gain, body fat and visceral adipose tissue (18–22), this was not observed in the current study. Specifically, there were no significant differences in body weight, total body fat or individual fat depots in male C57Bl/6J mice when fed a high-fat diet supplemented with 1% MOS. There are several aspects of this study that contrast with previous reports. This study utilized a purified MOS compound of measured chemical structure and substance. Previous studies have utilized a coffee-based MOS, derived from a galactomannan (18–22). Although the konjac-derived MOS used in this study has a different composition of monosaccharides constituents (mannose and glucose), the similar chemical structures of the mannan backbones should retain the undigestible β-1,4 glycoside linkages and produce similar fiber-like metabolic effects based on the proposed mechanism of action of MOS. However, the different monosaccharide composition of the MOS oligos was not directly tested and cannot be excluded as a contributor to the difference in observed results. Additionally, the purity of the MOS used in previous studies has not been disclosed. The amount used in this study should provide at least an equivalent amount of MOS in the diet that has been previously demonstrated as beneficial in mice (20).
Another point of contrast regards the mice used. This study utilized male C57Bl/6J strain, contrasted with the ICR female mice previously reported (20), raising the possibility that sex or strain differences contribute to the difference in results. However, C57Bl/6J mice are commonly used for diet-induced obesity conditions, as well as CR studies, and should serve as a useful model for this type of analysis.
Of particular note, an earlier study utilized a higher fat diet (63% of calories from fat) that was not reported to contain any fiber (20), compared with the normal fiber percentage (5% by weight) included in rodent diets. In contrast, the additional 1% MOS supplementation in this study provided a relative fiber increase of only 20% (from 5 g/100g diet to 6g/100g). This, in combination with a lower fat content in the current study (45% vs. 63%), may reduce any basic fiber effects previously observed. However, the results presented here should be relevant to more “normal” diets that contain at least some amount of fiber.
The food allotment of 95% AL was chosen to reduce variability in food intake values between animals and across groups in order to better assess the influence of MOS supplementation in the absence of confounding differences in food intake amounts (12). This was of particular interest in assessing the future use of MOS as a CR mimetic. Although increased food intake would not be measurable with this feeding paradigm, previous research reported significantly reduced body weight and body fat despite greater food intake with MOS supplementation (20). Therefore, any potential effect on body weight or body fat reduction would be larger when increased food intake is not permitted. In addition, multiple mice in both the CON and MOS groups did not fully consume their daily allotment during the progression of the study, suggesting compensatory feeding was not elevated with MOS supplementation or stimulated with advancing age and increasing body weight.
Despite the lack of a significant difference in body weight (Fig. 1.B.), MOS is proposed to reduce body fat accumulation that could be offset by gains in lean mass (20). However, body composition was measured every three weeks during the study and no significant differences were observed with MOS supplementation (Fig. 2). MOS supplementation has also been reported to selectively reduce visceral adipose depots (18, 21, 22). To address the potential that visceral adipose depots had been reduced while other depots increased, resulting in no difference in body weight or total body fat, individual fat pads were dissected and wet weights measured. There were no significant differences in individual, visceral or total fat pad weights (Fig. 2.D.), dismissing the possibility that MOS supplementation had selectively altered fat accumulation in this study. In addition to lean and fat, no significant differences were observed in the amount or density of bone between the groups. Although other types of non-digestible oligosaccharides have been shown to increase mineral absorption and promote bone production (32, 34), the MOS utilized in the current study did not.
During the study the mice seemed to tolerate the MOS supplementation, gaining similar amounts of body weight as the control group, with no adverse events observed. However, the lack of any reduction in body weight, body fat or visceral fat challenges the potential use of MOS as a CR mimetic or body composition enhancer.
Supplementary Material
Acknowledgments
We thank Dr. Xingsheng Li for assistance with acquisition of the MOS, Dr. David G. Pritchard for help with gas chromatography analysis, Dr. Maria S. Johnson for help with the body composition measurements, and Dr. Richard Weindruch for scientific advice. This research was supported in part by NIH grants P50AT00477, P30DK56336, P60DK079626 and T32DK062710. The opinions expressed herein are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated.
Footnotes
Disclosure statements:
Dr. Allison has received grants, consulting fees, and donations from multiple profit and nonprofit entities with interests in obesity including food companies which have interests in fiber and MOS.
Supplementary Material is available at www.nature.com/obesity.
References
- 1.Van Itallie TB. Obesity: adverse effects on health and longevity. Am J Clin Nutr. 1979;32(12 Suppl):2723–33. doi: 10.1093/ajcn/32.12.2723. [DOI] [PubMed] [Google Scholar]
- 2.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Kral JG, Naslund E. Surgical treatment of obesity. Nat Clin Pract Endocrinol Metab. 2007;3(8):574–83. doi: 10.1038/ncpendmet0563. [DOI] [PubMed] [Google Scholar]
- 4.Hofbauer KG, Nicholson JR, Boss O. The obesity epidemic: current and future pharmacological treatments. Annu Rev Pharmacol Toxicol. 2007;47:565–92. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- 5.McCay CM, Crowell MF. Prolonging the life span. Sci Mon. 1934;39:405–414. [Google Scholar]
- 6.McCay CM, Crowell MF. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 7.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35(3):299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 8.Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7(3):438–40. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf G. Calorie restriction increases life span: a molecular mechanism. Nutr Rev. 2006;64(2 Pt 1):89–92. doi: 10.1301/nr.2006.feb.89-92. [DOI] [PubMed] [Google Scholar]
- 10.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42(8):709–12. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Physiol Behav. 2008;94(5):643–8. doi: 10.1016/j.physbeh.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20(2):157–65. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 13.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: C. C. Thomas Publisher; 1988. [Google Scholar]
- 14.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–54. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 15.Ingram DK, Anson RM, de CR, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–23. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 16.Roth GS, Lane MA, Ingram DK. Caloric restriction mimetics: the next phase. Ann N Y Acad Sci. 2005;1057:365–71. doi: 10.1196/annals.1356.027. [DOI] [PubMed] [Google Scholar]
- 17.Hooge DM. Turkey Pen Trials with Dietary Mannan Oligosaccharide: Meta-analysis, 1993–2003. Int J Poultry Sci. 2004;3:179–188. [Google Scholar]
- 18.Kumao T, Fujii S. Mannooligosaccharides blended coffee beverage intake increases the fat level in feces. Journal of Health Science. 2006;52(3):329–32. [Google Scholar]
- 19.Kumao T, Fujii S, Asakawa A, Takehara I, Fukuhara I. Effect of coffee drink containing mannooligosaccharides on total amount of excreted fat in healthy adults. Journal of Health Science. 2006;52(4):482–5. [Google Scholar]
- 20.Takao I, Fujii S, Ishii A, et al. Effects of mannooligosaccharides from coffee mannan on fat storage in mice fed a high fat diet. Journal of Health Science. 2006;52(3):333–7. [Google Scholar]
- 21.Asano I, Fujii S, Ozaki K, Takehara I, Yi N, Fukuhara I. Effects of "Coffee Beverage" containing mannooligosaccharides from coffee on human abdominal fat by long term ingestion. Jap J Food Engineering. 2005;6:133–141. [Google Scholar]
- 22.Asano I, Fujii S, Kanost MR, Takehara I, Fukuhara I. Investigation of mannooligosaccharides blended coffee beverage on abdominal fat reduction in humans. Jap J Med Pharm Sci. 2006;55:93–103. [Google Scholar]
- 23.Moreira LR, Filho EX. An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol. 2008;79(2):165–78. doi: 10.1007/s00253-008-1423-4. [DOI] [PubMed] [Google Scholar]
- 24.Vuksan V, Sievenpiper JL, Xu Z, et al. Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr. 2001;20(5 Suppl):370S–80S. doi: 10.1080/07315724.2001.10719170. [DOI] [PubMed] [Google Scholar]
- 25.Asano I, Nakamura Y, Hoshino H, et al. Use of mannooligosaccharides from coffee mannan by intestinal bacteria. Nippon Nogeikagaku Kaishi-Journal of the Japan Society for Bioscience Biotechnology and Agrochemistry. 2001;75(10):1077–83. [Google Scholar]
- 26.Asano I, Hamaguchi K, Fujii S, Iino H. In vitro digestibility and fermentation of mannooligosaccharides from coffee mannan. Food Sci Technol Res. 2003;9:62–66. [Google Scholar]
- 27.Asano I, Umemura M, Fujii S, Hoshino H, Iino H. Effects of mannooligosaccharides from coffee mannan on fecal microflora and defecation in healthy volunteers. Food Sci Technol Res. 2004;10:93–97. [Google Scholar]
- 28.Umemura M, Fujii S, Asano I, Hoshino H, Iino H. Effect of small dose of mannooligosaccharides from coffee mannan on defecating conditions and fecal microflora. Food Sci Technol Res. 2004;10:174–179. [Google Scholar]
- 29.Umemura M, Fujii S, Asano I, Hoshino H, Iino H. Effect of coffee mix drink containing mannooligosaccharides from coffee mannan on defecation and fecal microbiota in healthy volunteers. Food Sci Technol Res. 2004;10:195–198. [Google Scholar]
- 30.Chen HL, Cheng HC, Wu WT, Liu YJ, Liu SY. Supplementation of konjac glucomannan into a low-fiber Chinese diet promoted bowel movement and improved colonic ecology in constipated adults: a placebo-controlled, diet-controlled trial. J Am Coll Nutr. 2008;27(1):102–8. doi: 10.1080/07315724.2008.10719681. [DOI] [PubMed] [Google Scholar]
- 31.Keithley J, Swanson B. Glucomannan and obesity: a critical review. Altern Ther Health Med. 2005;11(6):30–4. [PubMed] [Google Scholar]
- 32.Scholz-Ahrens KE, Schrezenmeir J. Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J Nutr. 2007;137(11 Suppl):2513S–23S. doi: 10.1093/jn/137.11.2513S. [DOI] [PubMed] [Google Scholar]
- 33.Scholz-Ahrens KE, Ade P, Marten B, et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr. 2007;137(3 Suppl 2):838S–46S. doi: 10.1093/jn/137.3.838S. [DOI] [PubMed] [Google Scholar]
- 34.Weaver CM. Inulin, oligofructose and bone health: experimental approaches and mechanisms. Br J Nutr. 2005;93(Suppl 1):S99–103. doi: 10.1079/bjn20041358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.